Peer-review started: August 12, 2016
First decision: September 30, 2016
Revised: October 12, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: January 6, 2017
Dent’s disease is an X-linked renal tubulopathy characterized by low molecular weight proteinuria, hypercalciuria and progressive renal failure. Disease aetiology is associated with mutations in the CLCN5 gene coding for the electrogenic 2Cl-/H+ antiporter chloride channel 5 (CLC-5), which is expressed in the apical endosomes of renal proximal tubules with the vacuolar type H+-ATPase (V-ATPase). Initially identified as a member of the CLC family of Cl- channels, CLC-5 was presumed to provide Cl- shunt into the endosomal lumen to dissipate H+ accumulation by V-ATPase, thereby facilitating efficient endosomal acidification. However, recent findings showing that CLC-5 is in fact not a Cl- channel but a 2Cl-/H+ antiporter challenged this classical shunt model, leading to a renewed and intense debate on its physiological roles. Cl- accumulation via CLC-5 is predicted to play a critical role in endocytosis, as illustrated in mice carrying an artificial Cl- channel mutation E211A that developed defective endocytosis but normal endosomal acidification. Conversely, a recent functional analysis of a newly identified disease-causing Cl- channel mutation E211Q in a patient with typical Dent’s disease confirmed the functional coupling between V-ATPase and CLC-5 in endosomal acidification, lending support to the classical shunt model. In this editorial, we will address the current recognition of the physiological role of CLC-5 with a specific focus on the functional coupling of V-ATPase and CLC-5.
Core tip: Chloride channel 5 (CLC-5) mutations cause Dent’s disease, which is characterized by renal proximal tubulopathy due to defective endocytosis. Recent revelations that CLC-5 is a 2Cl-/H+ antiporter and not a Cl- channel challenged the classical model proposing CLC-5 as a Cl- shunt to facilitate V-ATPase-mediated endosomal acidification. Therefore, physiological roles of CLC-5 and its interaction with V-ATPase in endosomal acidification and/or endocytosis are intensely debated. Recent functional analysis of a novel pure Cl- channel mutant from a Dent’s disease patient indicated a possible functional coupling between V-ATPase and CLC-5 not only in endosomal acidification but also at the plasma membrane.
- Citation: Satoh N, Suzuki M, Nakamura M, Suzuki A, Horita S, Seki G, Moriya K. Functional coupling of V-ATPase and CLC-5. World J Nephrol 2017; 6(1): 14-20
- URL: https://www.wjgnet.com/2220-6124/full/v6/i1/14.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i1.14
Renal proximal tubular cells have a high capacity for the uptake of various proteins in primary urine via receptor-mediated endocytosis. Acidified condition in intracellular organelles such as endosomes and lysosomes is essential for the normal endocytic pathway consisting of ligand-receptor dissociation, recycling of the uncoupled receptors to the cell surface and transport of the released ligands to lysosomes for degradation[1,2].
Intravesicular acidification is achieved mainly by the active H+-pumping of vacuolar ATPase (V-ATPase)[1,3]. Conversely, chloride channel 5 (CLC-5), long believed to be a pure chloride channel as the name implies, colocalizes with V-ATPase and is suggested to be involved in V-ATPase-mediated endosomal acidification by shunting Cl- to neutralize the positive charge due to H+ accumulation[4]. Indeed, mutations in CLC-5 cause Dent’s disease, which is characterized by renal proximal tubulopathy due to defective endocytosis[5,6], suggesting that V-ATPase and CLC-5 are functionally coupled in endosomes. However, recent reports demonstrating that CLC-5 is not a Cl- channel but in fact functions as a 2Cl-/H+ antiporter demands re-evaluation of its physiological roles and the pathogenesis of Dent’s disease[7-9]. Although the debate is ongoing on whether Cl- accumulation[10] or CLC-5-induced V-ATPase activation is more important for normal endocytosis, we recently demonstrated that impaired endosomal acidification derived from inadequate V-ATPase activation by mutated CLC-5 may be the underlying pathology in Dent’s disease[11]. Moreover, we identified such functional relationship between V-ATPase and CLC-5 even at the plasma membrane of mouse proximal tubules.
CLC-5, a member of the CLC family, was originally identified by cloning of a voltage-gated chloride channel, CLC-0, from Torpedo marmorata electric organ[12]. Several human mutations in corresponding genes of the CLC family are known to cause genetic disorders such as myotonia congenita (CLC-1), Barter syndrome (CLC-Kb), osteopetrosis (CLC-7) and Dent’s disease (CLC-5)[13,14].
Dent’s disease is an X-linked proximal renal tubulopathy arising from mutations in the CLCN5 gene encoding for the electrogenic 2Cl-/H+ antiporter CLC-5[7-9] and is characterized by low molecular weight (LMW) proteinuria, hypercalciuria, nephrocalcinosis, nephrolithiasis and slowly progressive renal failure[15,16]. In addition, affected patients present with various clinical signs of proximal tubular dysfunction including glycosuria, aminoaciduria, hyperphosphaturia and uricosuria, which is consistent with partial Fanconi syndrome[5]. These manifestations usually remain subtle or asymptomatic during childhood; however, Dent’s disease leads to chronic renal insufficiency over a few decades. Furthermore, in the absence of effective therapy, early diagnosis for Dent’s disease only allows for conservative therapy to prevent kidney stones and progression of chronic renal insufficiency[16].
CLC-5 is abundantly expressed in renal and intestinal epithelial cells, though it is also expressed in brain, lung and, to a lesser extent, liver[17]. In the kidney, CLC-5 expression is highest in proximal tubules and intercalated cells of the collecting ducts[18]. Especially in proximal tubules, a major site for urinary LMW protein reabsorption via receptor-mediated endocytosis[17,19], CLC-5 is predominantly located in early endosomes, colocalizing with V-ATPase[4,20]. Additionally, CLC-5 is also found to an extent in the apical membrane of proximal tubules, as suggested by its robust plasma membrane expression in HEK293 cells as well as in Xenopus laevis oocytes following heterologous overexpression[4,18].
V-ATPase is a large multi-subunit complex that is ubiquitously expressed in intracellular organelles of eukaryotic cells such as endosomes, lysosomes, secretary granules and trans-Golgi network[21,22]. V-ATPase pumps H+ across membranes using energy generated by ATP hydrolysis and provides an acidic intraorganellar environment that is critical for normal endocytic pathway[22-24]. Thus, V-ATPase lowers luminal pH of early endosomes and promotes the dissociation of internalized ligand–receptor complexes, which is essential for both recycling of the uncoupled receptors to cell surface and transport of the released ligands first to late endosomes and then to lysosomes for protein degradation[24-27].
In addition to intracellular organelles, V-ATPase is also highly expressed in plasma membrane of specialized cells in kidney and other tissues. Though V-ATPase is found over the entire length of the nephron, it is abundantly expressed in renal proximal tubular cells[2,28,29] and intercalated cells of the collecting duct[21,29,30], playing a pivotal role in acid-base homeostasis.
Proximal tubular cells secrete H+ from cytosol to tubular lumen via Na+/H+ exchangers (NHEs), mainly NHE3[31] on apical membrane, and reabsorb approximately 80% of the filtered HCO3-[32-34]. V-ATPase is assumed to be partially involved in this reabsorption process along the proximal tubules[26,35,36]. In the collecting duct, alpha-intercalated cells secrete H+ into the urine via apical membrane V-ATPase, while beta-intercalated cells export H+ into the vessel lumen via basolateral membrane V-ATPase, regulating final urine acidification. Genetic defects in specific V-ATPase subunits are known to cause renal tubular acidosis (RTA)[37-39]. Specifically, mutations in ATP6V0A4 and ATP6V0B1 coding for a4 and B1 subunits, respectively, lead to distal RTA in humans[38,40].
While the conventional functions of intracellular V-ATPase are well established, recent studies suggested that V-ATPase might have noncanonical functions as well[41]. For example, V-ATPase achieves the desired acidic endosomal pH in order to regulate the budding of endosomal carrier vesicles, where V-ATPase itself functions as not only a proton pump but also a pH sensor[1,42,43]. Luminal pH information is hypothesized to be detected by V-ATPase and transmitted to the cytosolic side via conformational changes in its transmembrane a2 isoform. This in turn results in the recruitment of ADP-ribosylation factor (ARF)-6 (ARF-6) and ARF-nucleotide binding-site opener (ARNO), both of which are involved in endocytosis by regulating the formation of endosomal carrier vesicles. Conversely, lysosomal V-ATPase is also suggested to constitute an important component of the lysosomal-associated amino acid sensing machinery[44]. Although the precise mechanism underlying this function of V-ATPase is unknown, accumulation of amino acids in lysosomes activates Rag guanosine triphosphatases (GTPases)[45] that promote the translocation of the master growth regulator mechanistic target of rapamycin complex 1 (mTORC1) to the lysosomal membrane[46,47]. In this process, the interaction between V-ATPase and the regulator that anchors Rag GTPases to the lysosomes is necessary for amino acid-induced signal transduction[48]. Therefore, lysosomal V-ATPase performs pivotal roles as both a proton pump and an amino acid sensor transmitting signals to activate mTORC1, which is essential for lysosomal function[49].
As indicated above, active H+ pumping by V-ATPase contributes to the preservation of an acidic luminal pH within intracellular organelles including endosomes and lysosomes, which is required for normal endocytic process. In contrast, two different strains of CLC-5 knockout mice developed LMW proteinuria, typical symptoms of Dent’s disease due to defective endocytosis[50,51]. Furthermore, Günther et al[6] showed that endosomes isolated from CLC-5 knockout mice were acidified at a significantly lower rate and to a lesser extent than those from wild-type mice. Subsequent analyses verified the lower luminal concentrations of Cl- and H+ in early endosomes isolated from proximal tubules of CLC-5 knockout mice[52]. These observations, in conjunction with the colocalization of CLC-5 with V-ATPase in early endosomes, strongly suggest that V-ATPase and CLC-5 are functionally coupled during endosomal acidification and/or endocytosis.
Indeed, since its identification as a member of the CLC family, CLC-5 was considered to be a Cl- channel[53] that provided Cl- to counter and dissipate positive charge (H+) accumulation generated by V-ATPase, thereby facilitating efficient endosomal acidification. This Cl- shunt model facilitated by functional V-ATPase coupling with CLC-5 was considered essential for normal endocytosis[25]. However, recent studies demonstrating that CLC-5 is not a Cl- channel but a 2Cl-/H+ antiporter forced comprehensive reevaluation of these physiological roles of CLC-5[7-9], given that the 2Cl-/H+ antiporter would result in ineffective acidification due to parallel H+ efflux at the expense of wasted energy (ATP) by V-ATPase. Thus, physiological roles of CLC-5 as a 2Cl-/H+ antiporter and its interaction with V-ATPase in endosomal acidification and/or endocytosis remain unknown and have become an important issue in the field.
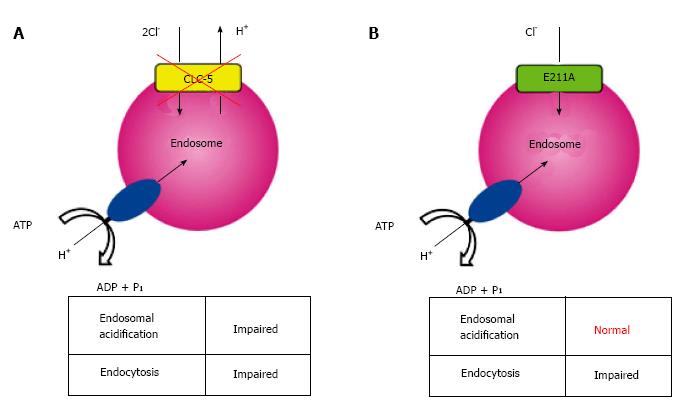
Analysis of a mutation in the so-called gating glutamate at position 211 (E211), a conserved residue that serves as a central gate for H+-coupled Cl- transport[54,55], may provide a key in understanding the complex nature of CLC-5 functions. Novarino and colleagues generated mice carrying the E211A mutation which deprived H+ transport of CLC-5, altering it to a simple Cl- conductance[10], and these mice developed defective endocytosis similar to that observed in CLC-5 knockout mice. Surprisingly, however, endosomal acidification was preserved in E211A mice in contrast to CLC-5 knockout mice (Figure 1). Therefore, they proposed that endosomal Cl- accumulation rather than endosomal acidification might be critical for renal endocytosis. However, mutations in the gating glutamate, such as that is present in E211A mutant mice, have not yet been identified in patients with Dent’s disease.
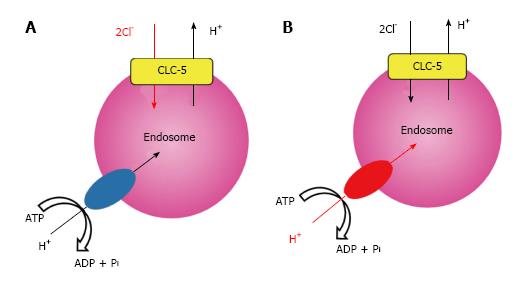
We recently analyzed a previously unrecognized mutation of E211 to glutamine (E211Q) that was found in a typical Dent’s disease patient[56] and confirmed that functional coupling between V-ATPase and CLC-5 occurred in endosomal acidification[11]. Electrophysiological studies in Xenopus laevis oocytes demonstrated that the disease-causing mutant E211Q had similar characteristics to the artificial mutant E211A. Thus, E211Q mutation also abolished H+ transport of CLC-5 and altered it to a simple Cl- conductance, which was supported by the molecular modelling of CLC-5 mutants[11]. Upon heterologous overexpression in HEK293 cells, both Cl- channel mutants, E211Q and E211A, enhanced bafilomycin-sensitive endosomal acidification. However, acidification was greater in endosomes expressing wild-type CLC-5. Because CLC-5-induced endosomal acidification reflected V-ATPase activity, these results indicated that the 2Cl-/H+ exchange mode of CLC-5 was required for maximal endosomal acidification[11]. Indeed, simulation studies on lysosomal acidification via CLC-7, another CLC member with Cl-/H+ exchange function, lent support to the model in which the Cl-/H+ exchange mode was more advantageous than the Cl- channel mode in intravesicular acidification[57,58]. Our results revealed that the conversion of CLC-5 from a 2Cl-/H+ antiporter into a Cl- conductance is the definite cause of Dent’s disease. More importantly, we showed that impaired endosomal acidification via inadequate V-ATPase activation should be considered as a pivotal component of the aetiology in Dent’s disease. These potential roles of CLC-5 in endosome are summarized in Figure 2.
Based on the accumulating data, the cause of discrepancy in endosomal acidification by Cl- channel mutants between mice proximal tubules and HEK293 cells remains unclear. Significant basal acidification was still found in endocytic vesicles isolated from CLC-5 knockout mouse kidneys[6]. In contrast, in the absence of endogenous CLC-5[59], the basal V-ATPase activity in HEK293 cells was quite subtle. Therefore, a small difference in endosomal acidification generated by the E211A mutant that was detectable in HEK293 cells might have been overlooked in proximal tubules obtained from E211A mice. Of course, genetically altered mice could have potentially developed compensatory mechanisms. However, a convincing model for the interaction between CLC-5 and V-ATPase in endosomes continues to be a focus of intense debate.
Physiological significance of CLC-5 at the plasma membrane is not clear[14]. Therefore, we also investigated the impact of CLC-5 on plasma membrane V-ATPase function by measuring hypotonicity-induced V-ATPase activity as previously reported[60,61]. Heterologous overexpression of both E211Q and E211A mutants in HEK293 cells led to the moderate activation of membrane V-ATPase. However, wild-type CLC-5 induced even higher V-ATPase activation, which was in harmony with the degree of endosomal acidification. This V-ATPase activation by hypotonicity was observed even in isolated mouse proximal tubules. Furthermore, siRNA-mediated gene silencing for CLC-5 strongly reduced V-ATPase activity, suggesting the presence of tight functional coupling between V-ATPase and CLC-5 even in apical membrane of intact proximal tubules. Although the detailed mechanisms by which CLC-5 activates the membrane V-ATPase are unknown, noncanonical roles of V-ATPase may allow several possible explanations. In addition to the abovementioned multiple V-ATPase functions, it is known that CLC-5 mediates the assembly with other proteins, and several binding proteins have already been proposed[14]. Therefore, it is possible that the 2Cl-/H+ exchange mode of CLC-5 induces V-ATPase activation by recruiting unknown cellular factors and/or by directly modifying the function of V-ATPase[11].
Loss-of-function mutations in CLC-5 were definitely shown to cause Dent’s disease phenotypes in humans as well as in mouse models, suggesting the indispensability of CLC-5 for normal endocytic pathway. However, it remains unclear whether Cl- accumulation or V-ATPase-mediated acidification by CLC-5 is more important for normal endocytosis. Our recent study focusing on disease-causing mechanisms of the E211Q mutant of CLC-5 revealed that impaired endosomal acidification caused by inadequate CLC-5-induced V-ATPase activation may play a key role in the aetiology of Dent’s disease. However, future studies are necessary to clarify the potentially critical role of endosomal Cl- accumulation, as suggested by the findings in mice carrying the E211A mutation.
We wish to acknowledge Drs. Akira Ashida, Daisuke Yamamoto, Yoshitsugu Kaku and Takashi Sekine for their valuable contribution to our previous work.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Watanabe T, Yorioka N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1057] [Cited by in F6Publishing: 1093] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 2. | Jefferies KC, Cipriano DJ, Forgac M. Function, structure and regulation of the vacuolar (H+)-ATPases. Arch Biochem Biophys. 2008;476:33-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Nishi T, Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 926] [Cited by in F6Publishing: 893] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 4. | Günther W, Lüchow A, Cluzeaud F, Vandewalle A, Jentsch TJ. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci USA. 1998;95:8075-8080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 318] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Wrong OM, Norden AG, Feest TG. Dent’s disease; a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. QJM. 1994;87:473-493. [PubMed] [Cited in This Article: ] |
| 6. | Günther W, Piwon N, Jentsch TJ. The ClC-5 chloride channel knock-out mouse - an animal model for Dent‘s disease. Pflugers Arch. 2003;445:456-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Picollo A, Pusch M. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 2005;436:420-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 356] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 8. | Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 2005;436:424-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 387] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 9. | Zifarelli G, Pusch M. Conversion of the 2 Cl(-)/1 H+ antiporter ClC-5 in a NO3(-)/H+ antiporter by a single point mutation. EMBO J. 2009;28:175-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Novarino G, Weinert S, Rickheit G, Jentsch TJ. Endosomal chloride-proton exchange rather than chloride conductance is crucial for renal endocytosis. Science. 2010;328:1398-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Satoh N, Yamada H, Yamazaki O, Suzuki M, Nakamura M, Suzuki A, Ashida A, Yamamoto D, Kaku Y, Sekine T. A pure chloride channel mutant of CLC-5 causes Dent’s disease via insufficient V-ATPase activation. Pflugers Arch. 2016;468:1183-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 376] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Jentsch TJ, Poët M, Fuhrmann JC, Zdebik AA. Physiological functions of CLC Cl- channels gleaned from human genetic disease and mouse models. Annu Rev Physiol. 2005;67:779-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Stauber T, Weinert S, Jentsch TJ. Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol. 2012;2:1701-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Claverie-Martín F, Ramos-Trujillo E, García-Nieto V. Dent’s disease: clinical features and molecular basis. Pediatr Nephrol. 2011;26:693-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Devuyst O, Thakker RV. Dent’s disease. Orphanet J Rare Dis. 2010;5:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J Biol Chem. 1995;270:31172-31177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 231] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Sayer JA, Stewart GS, Boese SH, Gray MA, Pearce SH, Goodship TH, Simmons NL. The voltage-dependent Cl(-) channel ClC-5 and plasma membrane Cl(-) conductances of mouse renal collecting duct cells (mIMCD-3). J Physiol. 2001;536:769-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Pusch M, Zifarelli G. ClC-5: Physiological role and biophysical mechanisms. Cell Calcium. 2015;58:57-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Sakamoto H, Sado Y, Naito I, Kwon TH, Inoue S, Endo K, Kawasaki M, Uchida S, Nielsen S, Sasaki S. Cellular and subcellular immunolocalization of ClC-5 channel in mouse kidney: colocalization with H+-ATPase. Am J Physiol. 1999;277:F957-F965. [PubMed] [Cited in This Article: ] |
| 21. | Brown D, Paunescu TG, Breton S, Marshansky V. Regulation of the V-ATPase in kidney epithelial cells: dual role in acid-base homeostasis and vesicle trafficking. J Exp Biol. 2009;212:1762-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Forgac M. Structure and properties of the vacuolar (H+)-ATPases. J Biol Chem. 1999;274:12951-12954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Bowman EJ, Bowman BJ. Cellular role of the V-ATPase in Neurospora crassa: analysis of mutants resistant to concanamycin or lacking the catalytic subunit A. J Exp Biol. 2000;203:97-106. [PubMed] [Cited in This Article: ] |
| 24. | Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPases. FEBS Lett. 1998;440:258-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1498] [Cited by in F6Publishing: 1529] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 26. | Gluck S, Nelson R. The role of the V-ATPase in renal epithelial H+ transport. J Exp Biol. 1992;172:205-218. [PubMed] [Cited in This Article: ] |
| 27. | Sun-Wada GH, Wada Y. Vacuolar-type proton pump ATPases: acidification and pathological relationships. Histol Histopathol. 2013;28:805-815. [PubMed] [Cited in This Article: ] |
| 28. | Al-bataineh MM, Gong F, Marciszyn AL, Myerburg MM, Pastor-Soler NM. Regulation of proximal tubule vacuolar H(+)-ATPase by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol. 2014;306:F981-F995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Biner HL, Arpin-Bott MP, Loffing J, Wang X, Knepper M, Hebert SC, Kaissling B. Human cortical distal nephron: distribution of electrolyte and water transport pathways. J Am Soc Nephrol. 2002;13:836-847. [PubMed] [Cited in This Article: ] |
| 31. | Wu MS, Biemesderfer D, Giebisch G, Aronson PS. Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem. 1996;271:32749-32752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol. 2006;17:2368-2382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Vallon V, Schwark JR, Richter K, Hropot M. Role of Na(+)/H(+) exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol. 2000;278:F375-F379. [PubMed] [Cited in This Article: ] |
| 34. | Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol. 1999;277:F298-F302. [PubMed] [Cited in This Article: ] |
| 35. | Chan YL, Giebisch G. Relationship between sodium and bicarbonate transport in the rat proximal convoluted tubule. Am J Physiol. 1981;240:F222-F230. [PubMed] [Cited in This Article: ] |
| 36. | Zimolo Z, Montrose MH, Murer H. H+ extrusion by an apical vacuolar-type H(+)-ATPase in rat renal proximal tubules. J Membr Biol. 1992;126:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Escobar L, Mejía N, Gil H, Santos F. Distal renal tubular acidosis: a hereditary disease with an inadequate urinary H excretion. Nefrologia. 2013;33:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 38. | Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 470] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 39. | Zhang J, Fuster DG, Cameron MA, Quiñones H, Griffith C, Xie XS, Moe OW. Incomplete distal renal tubular acidosis from a heterozygous mutation of the V-ATPase B1 subunit. Am J Physiol Renal Physiol. 2014;307:F1063-F1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Karet FE, Finberg KE, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Medina JF, Lifton RP. Localization of a gene for autosomal recessive distal renal tubular acidosis with normal hearing (rdRTA2) to 7q33-34. Am J Hum Genet. 1999;65:1656-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Maxson ME, Grinstein S. The vacuolar-type H+-ATPase at a glance - more than a proton pump. J Cell Sci. 2014;127:4987-4993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 42. | Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8:124-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 372] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 43. | Marshansky V. The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem Soc Trans. 2007;35:1092-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1211] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 45. | Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 940] [Cited by in F6Publishing: 1007] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 46. | Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med. 2012;18:524-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 47. | Hu Y, Carraro-Lacroix LR, Wang A, Owen C, Bajenova E, Corey PN, Brumell JH, Voronov I. Lysosomal pH Plays a Key Role in Regulation of mTOR Activity in Osteoclasts. J Cell Biochem. 2016;117:413-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1829] [Cited by in F6Publishing: 1773] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 49. | Puertollano R. mTOR and lysosome regulation. F1000Prime Rep. 2014;6:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ. ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent‘s disease. Nature. 2000;408:369-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 51. | Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB. Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet. 2000;9:2937-2945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 252] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Hara-Chikuma M, Wang Y, Guggino SE, Guggino WB, Verkman AS. Impaired acidification in early endosomes of ClC-5 deficient proximal tubule. Biochem Biophys Res Commun. 2005;329:941-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Friedrich T, Breiderhoff T, Jentsch TJ. Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J Biol Chem. 1999;274:896-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 54. | Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 646] [Cited by in F6Publishing: 650] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 55. | Yin J, Kuang Z, Mahankali U, Beck TL. Ion transit pathways and gating in ClC chloride channels. Proteins. 2004;57:414-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Sekine T, Komoda F, Miura K, Takita J, Shimadzu M, Matsuyama T, Ashida A, Igarashi T. Japanese Dent disease has a wider clinical spectrum than Dent disease in Europe/USA: genetic and clinical studies of 86 unrelated patients with low-molecular-weight proteinuria. Nephrol Dial Transplant. 2014;29:376-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Ishida Y, Nayak S, Mindell JA, Grabe M. A model of lysosomal pH regulation. J Gen Physiol. 2013;141:705-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Weinert S, Jabs S, Supanchart C, Schweizer M, Gimber N, Richter M, Rademann J, Stauber T, Kornak U, Jentsch TJ. Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl- accumulation. Science. 2010;328:1401-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 59. | Smith AJ, Lippiat JD. Direct endosomal acidification by the outwardly rectifying CLC-5 Cl(-)/H(+) exchanger. J Physiol. 2010;588:2033-2045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Amlal H, Goel A, Soleimani M. Activation of H+-ATPase by hypotonicity: a novel regulatory mechanism for H+ secretion in IMCD cells. Am J Physiol. 1998;275:F487-F501. [PubMed] [Cited in This Article: ] |
| 61. | Rahmati N, Kunzelmann K, Xu J, Barone S, Sirianant L, De Zeeuw CI, Soleimani M. Slc26a11 is prominently expressed in the brain and functions as a chloride channel: expression in Purkinje cells and stimulation of V H+-ATPase. Pflugers Arch. 2013;465:1583-1597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |