Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2019; 28(2): 279-288
Published online April 30, 2019
https://doi.org/10.5607/en.2019.28.2.279
© The Korean Society for Brain and Neural Sciences
miR-381 Attenuates Peripheral Neuropathic Phenotype Caused by Overexpression of PMP22
Ji-Su Lee1, Geon Kwak1, Hye Jin Kim1, Hwan-Tae Park2, Byung-Ok Choi1,3*, and Young Bin Hong4*
1Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul 06351, Korea.
2Department of Physiology, College of Medicine, Dong-A University, Busan 49201, Korea.
3Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea.
4Department of Biochemistry, College of Medicine, Dong-A University, Busan 49201, Korea.
Correspondence to: *To whom correspondence should be addressed.
Young Bin Hong, TEL: 82-51-240-2762, FAX: 82-51-240-2971
e-mail: ybhong@dau.ac.kr
Byung-Ok Choi, TEL: 82-2-3410-1296, FAX: 82-2-3410-0052
e-mail: bochoi@skku.edu
Abstract
Charcot-Marie Tooth disease type 1A (CMT1A), the major type of CMT, is caused by duplication of peripheral myelin protein 22 (
Graphical Abstract
Keywords: Peripheral myelin protein 22 (PMP22), Demyelination, microRNA (miRNA), Charcot-Marie-Tooth disease (CMT)
INTRODUCTION
Charcot-Marie-Tooth disease (CMT) is one of major inherited peripheral neuropathies with various genetic and clinical heterogeneity [1]. So far, up to 80 genes have been isolated as causative genes [2]. Among them, duplication of peripheral myelin protein 22 (
MicroRNAs (miRNA) are endogenous small noncoding RNAs that are present as RNA-duplex transcripts of approximately 22 nucleotides in length [10]. The significance of regulatory function of miRNAs in development and cellular homeostasis has been demonstrated [11]. During the development of peripheral nervous system, the importance of miRNAs has been investigated. Ablation of Dicer from Schwan cells can block normal myelination and axonal integrity [12,13,14]. In addition, reduction of Dicer impairs Schwann cell differentiation and myelination [15]. Regarding PMP22 gene expression, several miRNAs such as miR-9 and miR-29b are known to target 3′untranslated region (3′UTR) of
Thus, the objective of the present study was to investigate the potency of miRNA as a therapeutic strategy for controlling CMT1A, a disease with copy number variation in peripheral neuropathy. We identified a candidate miRNA whose expression was lowered in CMT1A mouse model. It showed potency in reducing the expression of target gene, PMP22. We also evaluated the potency of miRNA in attenuating the phenotype of CMT1A by regulating the expression level of PMP22 through in vivo study.
MATERIALS AND METHODS
Expression level of miRNA was evaluated by RNA-sequencing based small RNA sequencing performed by Macrogen, Inc. (Seoul, Korea). TruSeq small RNA library prep kit (Illumina, San Diego, CA, USA) was used after isolating total RNAs from sciatic nerve of wild-type and C22 mice (n=3). MicroRNAs and a control miRNA were synthesized by GenePharma (Shanghai, China).
To obtain PMP22 and PMP22-3′UTR, human total mRNA and gDNA were used as templates for PCR amplification. Amplified gene was cloned into pCMV-myc vector (Clontech, Mountain View, CA, USA). For lentiviral expression of miRNAs, LV-mock (miRNA mimic negative control) and LV-miR381 or miR-9 expression cassette were generated based on lentivirus vector pLVX-IRES-Zsgreen1 (Clontech, Mountain View, CA, USA). Lentiviral particles were produced in 4.0×106 HEK293T cells by co-transfection of lentiviral expression plasmids and packaging plasmids from SIRION Biotech (Martinsried, Germany). Supernatant was harvested at 48h after transfection. Lentiviral particles were precipitated with polyethylene glycol and dissolved in 400 µl serum free CD29 medium (Life Technologies, Carlsbad, CA, USA).
All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committees of Samsung Medical Center (SMC-20170203001). C22 mouse [B6;CBACa-Tg(PMP22)C22Clh/H] was purchased from MRC Harwell (Oxfordshire, UK). The C22 mouse is one of the most frequently used mouse model of CMT1A. The mice harbors 7 copies of human PMP22 genes and expression level of hPMP22 is 1.7 fold higher than mPMP22 in the sciatic nerve, which cause severe demyelinating neuropathy within 3 weeks of age [17,18,19,20,21]. Lentiviral particles (7.5×104 IU/mouse) were intraneurally injected distal to the sciatic notch at postnatal day 6 (p6) as previously described [22]. A 10 µl Hamilton syringe connected to a 33-gauge needle was used for intraneural injection. Motor coordination was assessed using a Rotarod apparatus (B.S. Technolab INC, Seoul, Korea) with a horizontal rotating rod (21 rpm). The holding time of animals on the rotating rod was measured. Mice were allowed to stay on the rod for a maximum of 300 seconds.
To assess electrophysiological status, a nerve conduction study (NCS) was performed for wild-type (WT) and C22 mice at 10 weeks post administration as previously described [23]. Briefly, mice were anesthetized with 1.5% isoflurane supplied using a nose cone for the duration of the procedure. NCS was performed using a Nicolet VikingQuest device (Natus Medical, San Carlos, CA, USA). For measurement of motor nerve conduction velocity (MNCV), the active recording needle electrode (cathode) was placed onto the gastrocnemius muscle with the reference electrode (anode) on its tendon, and the stimulating cathode was perpendicularly inserted approximately 2mm under the skin without direct contact to nerve, at the position of 6mm proximal to the recording electrode in the midline of the posterior thigh and 6 mm proximally in the medial gluteal region to obtain distal and proximal responses, respectively. The anode was subcutaneously placed in the midline over the sacrum. A surface electrode as a ground electrode placed on the mouse's tail. Finally, single square-wave pulses of 0.1-ms duration were delivered to obtain the conduction signal. Motor nerve conduction velocity (MNCV) and compound muscle action potential (CMAP) amplitudes at supramaximal stimulation were determined by an independent examiner who was blinded to genotype and treatment groups.
Sciatic nerves were biopsied from both WT and C22 mice at 15 weeks post LV-miR381 administration. Pathological examinations of affected specimens were performed through microscopic analyses. Specimens were fixed overnight with 2.5% glutaraldehyde in 4% paraformaldehyde solution at 4℃. After incubation for 1 hr in 1% OsO4, specimens were dehydrated in an ethanol series, passed through propylene oxide, and embedded in epoxy resin (Epok 812, Oken, Japan). Tissues were cut into semi-thin (1µm) sections and stained with toluidine blue for 5 to 10 seconds. Semi-thin sections were imaged using a Bx51 upright microscope (Olympus, Tokyo, Japan) and analyzed using Cell sense (Olympus). Ultrathin sections (65 nm) were collected on 200 mesh nickel grids and stained with 2% uranyl acetate for 15 min and lead citrate for 5 min. These specimens were observed with a Hitachi HT7700 electron microscope at 100 kV. About 300 axons were counted per animal.
Total RNA was extracted from Schwann cells or sciatic nerves of mice at 15 weeks post administration using RNeasy Mini Kit (Qiagen, Hilden, Germany). After reverse transcription using SuperScript™ II reverse transcriptase (Thermo Fisher, Rockford, IL, USA), the resulting cDNA was used as template for PCR amplification with the following primers: human PMP22-F, 5′-ATCGTCAGCCAATGGATCGTG-3′; human PMP22-R, 5′-AGAAACAGTGGTGGACATTTCC-3′; mouse actin-F, 5′-GTGACGTTGACATCCGTAAAGA-3′; mouse actin-R, 5′-GCCGGACTCATCGTACTCTCC-3′; rat GAPDH-F, 5′-TGCCACTCAGAAGACTGTGG-3′; and rat GAPDH-R, 5′-TTCAGCTCTGGGATGACCTT-3′. Real-time PCR reactions were performed using SYBR Green PCR master mix and ABI QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). To quantify levels of miRNA, qRT-PCR was performed using mature miRNA-specific TaqMan primers (Applied Biosystems).
Total proteins were collected after lysis with RIPA buffer (Biosesang, Seoul, Korea) for standard Western blotting. Antibodies used for the determination of proteins of interest were: anti-myc (Abcam, Cambridge, UK), anti-GAPDH, and anti-Rabbit IgG (Cell signaling Technology, Beverly, MA, USA). The band intensity was determined by the Image J program (https://imagej.nih.gov) then normalized to GAPDH. Immunohistochemistry was performed on sliced sections (4 µm) using anti-PMP22 antibodies (Abcam) and Alexa Fluor 594 secondary antibodies (Invitrogen, Life Technologies, Carlsbad, CA, USA). Quantification of protein level was acquired for arithmetic mean intensity with ZEN program.
All values are expressed as mean±standard error of the mean (SEM). For pairwise comparisons, statistical significance of data presented was evaluated by Student's t-test. For comparisons of more than 2 treatment groups, statistical significance of data presented was evaluated by 1-way ANOVA. The level of significance was set at p<0.05.
RESULTS
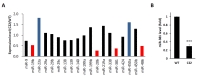
To address whether expression levels of miRNAs known to be involved in myelination were changed in CMT1A disease mouse model, the miRNA expression profile was analyzed using RNA-sequencing. Expression levels of miR-19b, miR-206, miR-381, and miR-486 in sciatic nerves of C22 mice (CMT1A animal model) were lower than those of wild-type mice (Fig. 1A). On the other hand, expression levels of miR-23a and miR-450a were elevated in C22 mice. Among these miRNAs, we focused on miR-381 because it was previously reported to be one of miRNAs with potency in targeting 3′UTR of
Since miR-381 was previously proposed to target 3′UTR of
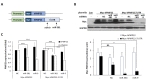
Next, we examined whether miR-381 could regulate expression level of PMP22 in sciatic nerves of C22 mice. To deliver miR-381 into the sciatic nerve, we cloned human miR-381 and generated lentiviral particle expressing miR-381 (LV-miR-381) under the control of CMVIE promoter. We then administered LV-miR-381 or control lentiviral vector (LV-mock) into sciatic nerve of C22 mouse model which carries 7 copies of human
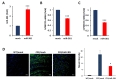
Next, we examined whether the reduction of PMP22 level by miR-381 was linked to amelioration of abnormal neuropathic phenotype of C22 mice. First, we analyzed the effect on locomotor coordination. Rotarod analysis showed that LV-miR-381 treatment prevented the progression of behavioral deficits which can be observed in C22 mice. C22 mice administered with LV-miR-381 sustained locomotor coordination through 15 weeks of age thereby exhibiting significantly higher performance from 9 weeks of ages (8 weeks post administration) (Fig. 4A). To link the enhancement of locomotor coordination in LV-miR-381 treated group to structural and functional improvement of peripheral nerve integrity, we evaluated nerve electrophysiology. Human PMP22 overexpressing C22 mice exhibited slow motor nerve conduction velocity (MNCV) and reduced compound action potential (CMAP) compared to wild-type mice [20]. Administration of LV-miR-381 significantly increased MNCV compared to LV-mock treated C22 mice at 10 weeks post administration (Fig. 4B). However, we could not observe significant change in CMAP after administration of LV-miR-381 (Fig. 4C).
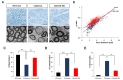
We then analyzed histopathological improvement by LV-miR-381 using semi-thin sections of sciatic nerves. Toluidine blue staining revealed that the number of myelinated axons, diameter of axons, and overall thickness of myelination of Schwann cells were decreased in C22 mice treated LV-mock compared to those in wild-type mice treated LV-mock. However, treatment of LV-miR-381 increased the number of myelinated axons and the thickness of myelination in C22 mice. Electron microscopic images also showed enhanced myelination pattern in LV-miR-381 treated mice (Fig. 5A). To quantitatively analyze toluidine blue staining images, we measured g-ratio, the ratio of inner axonal diameter to total outer diameter. Results showed that the g-ratio of LV-miR-381 treated C22 mice had a lower slope than that of LV-mock treated C22 mice, indicating reduction of aberrant myelination by the expression of miR-381 (Fig. 5B). Direct counting revealed that the reduced number of myelinated axon in C22 mice was recovered by expression of miR-381 (Fig. 5C and 5D). In addition, the number of onion bulb formation was reduced in LV-miR-381 administered C22 group (Fig. 5E). However, there was no significant difference in the diameter of myelinated fibers or axons between LV-miR-381 treated C22 mice and LV-mock treated C22 mice, although these diameters were smaller than those of wild-type mice (Fig. 5A and 5B). Collectively, these data indicate that the expression of miR-381 in the sciatic nerve can ameliorate the phenotypic symptoms of CMT1A mouse model.
DISCUSSION
In this study, we evaluated the therapeutic feasibility of miRNA in the peripheral neuropathy with copy number variation. We found that the expression level of miR-381 was lower in PMP22 overexpressing mouse model. In addition, miR-381 targeted 3′UTR of
To treat CMT1A, numerous attempts have been made using dietary supplements, growth factors, and chemical drugs. Ascorbic acid has received plausible attentions because of its feasibility and mode of action in reducing PMP22 expression through modulating cAMP level [6,25]. However, results from clinical trials on the efficacy of ascorbic acid drugs are questionable [8,9]. Growth factors such as neuregulin and neurotrophin-3 have also been evaluated for their efficacy in the modulation of disease phenotype in relation to improving myelination and axonal integrity [24,26,27,28]. Although these approaches have not reached a stage of clinical trials, they might be considered as potent therapeutic options after further development. The advent of systems biology has influenced drug repositioning approaches in CMT treatment. The combination of commercially available drugs (PXT3003) has been clinically evaluated in both animal model and patients [29,30]. Although these approaches are fundamental strategies to treat genetic diseases, they are based on indirect manipulation of protein expression, facilitation of myelination, or reducing cytotoxicity to nerves. To overcome genetic diseases, more direct approaches might be needed to manipulate duplicated genes. In this context, direct gene manipulation by editing or silencing the duplicated gene is worth trying for CMT1A. It has been recently reported that antisense oligonucleotide (ASO)-based downregulation of PMP22 is effective for rodent models of CMT1A [31]. Although ASO was administered after the onset of the disease, this therapy promoted the recovery of phenotypes of peripheral neuropathy as well as disease-associated gene expression network [31]. Previously, we have evaluated the efficacy of allele-specific small interfering RNA (siRNA) for targeting point mutation in
To expand therapeutic goals, we investigated the applicability of miRNA to treatment peripheral neuropathy. From the miRNA profile analysis, we found that expression level of several miRNAs such as miR-19b, miR-206 and miR-486 are decreased in sciatic nerve of CMT1A mice. Previously, miR-19b was reported to be consistently reduced during peripheral nerve development [33] and miR-206 is reduced in embryonic stem cells-derived oligodendrocyte differentiation [34]. miR-486 was proposed as a potential therapeutic target through the neuroprotection of NeuroD6 for spinal cord injuries [35]. However, the correlation of these miRNAs with CMT1A or PMP22 has not been reported. Since PMP22-mediated pathogenesis in CMT1A causes apoptosis or abnormal differentiation of Schwann cells in development stages, expression levels of myelination genes or their related genes might be affected in the CMT1A mouse model. Previously, miR-9 was proposed as to modulate the expression level of
In conclusion, we demonstrated that miRNA-mediated regulation of gene expression could be applied to treat diseases caused by copy number variation. Further investigations on roles and functions of miRNAs as well as its targets and related genetic environments with the development of its delivery systems might lead to its clinical application in peripheral neuropathy.
SUPPLEMENTARY MATERIAL
Suppl Fig 1
Enhancement of peripheral neuropathic phenotype by administration of miR-9. Lentiviruses expressing miR-9 (LV-miR-9, n = 5) or control lentivirus (LV-mock, n = 6) were intraneurally administered to six-day-old C22 mice pups. (A) Rotarod test from 3 weeks of age showing that C22 mice administered with LV-miR-9 exhibited significantly increased performance from 9 weeks. (B) Quantitative real-time PCR analysis of miR-9 from sciatic nerves of C22 mice showing efficient delivery of LV-miR-9. (C) Quantitative real-time PCR analysis of human PMP22 showing that its mRNA levels were decreased in LV-miR-9 treated mice compared to those in LV-mock treated mice. **, p < 0.01; ***, p < 0.001.
en-28-279-s001.pdfFigures

References
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin Genet 1974;6:98-118.
- Pareyson D, Saveri P, Pisciotta C. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Curr Opin Neurol 2017;30:471-480.
- Boerkoel CF, Takashima H, Garcia CA, Olney RK, Johnson J, Berry K, Russo P, Kennedy S, Teebi AS, Scavina M, Williams LL, Mancias P, Butler IJ, Krajewski K, Shy M, Lupski JR. Charcot-Marie-Tooth disease and related neuropathies: mutation distribution and genotype-phenotype correlation. Ann Neurol 2002;51:190-201.
- Dickson KM, Bergeron JJ, Shames I, Colby J, Nguyen DT, Chevet E, Thomas DY, Snipes GJ. Association of calnexin with mutant peripheral myelin protein-22
ex vivo : a basis for “gain-of-function” ER diseases. Proc Natl Acad Sci U S A 2002;99:9852-9857. - Sereda MW, Meyer zu Hörste G, Suter U, Uzma N, Nave KA. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat Med 2003;9:1533-1537.
- Passage E, Norreel JC, Noack-Fraissignes P, Sanguedolce V, Pizant J, Thirion X, Robaglia-Schlupp A, Pellissier JF, Fontés M. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat Med 2004;10:396-401.
- Meyer zu Horste G, Prukop T, Liebetanz D, Mobius W, Nave KA, Sereda MW. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann Neurol 2007;61:61-72.
- Pareyson D, Reilly MM, Schenone A, Fabrizi GM, Cavallaro T, Santoro L, Vita G, Quattrone A, Padua L, Gemignani F, Visioli F, Laurà M, Radice D, Calabrese D, Hughes RA, Solari A, Array. Ascorbic acid in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): a double-blind randomised trial. Lancet Neurol 2011;10:320-328.
- Lewis RA, McDermott MP, Herrmann DN, Hoke A, Clawson LL, Siskind C, Feely SM, Miller LJ, Barohn RJ, Smith P, Luebbe E, Wu X, Shy ME, Muscle Study Group. High-dosage ascorbic acid treatment in Charcot-Marie-Tooth disease type 1A: results of a randomized, double-masked, controlled trial. JAMA Neurol 2013;70:981-987.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-297.
- Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development 2005;132:4653-4662.
- Bremer J, O'Connor T, Tiberi C, Rehrauer H, Weis J, Aguzzi A. Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination. PLoS One 2010;5:e12450.
- Pereira JA, Baumann R, Norrmén C, Somandin C, Miehe M, Jacob C, Lühmann T, Hall-Bozic H, Mantei N, Meijer D, Suter U. Dicer in Schwann cells is required for myelination and axonal integrity. J Neurosci 2010;30:6763-6775.
- Yun B, Anderegg A, Menichella D, Wrabetz L, Feltri ML, Awatramani R. MicroRNA-deficient Schwann cells display congenital hypomyelination. J Neurosci 2010;30:7722-7728.
- Verrier JD, Semple-Rowland S, Madorsky I, Papin JE, Notterpek L. Reduction of Dicer impairs Schwann cell differentiation and myelination. J Neurosci Res 2010;88:2558-2568.
- Verrier JD, Lau P, Hudson L, Murashov AK, Renne R, Notterpek L. Peripheral myelin protein 22 is regulated posttranscriptionally by miRNA-29a. Glia 2009;57:1265-1279.
- Huxley C, Passage E, Manson A, Putzu G, Figarella-Branger D, Pellissier JF, Fontés M. Construction of a mouse model of Charcot-Marie-Tooth disease type 1A by pronuclear injection of human YAC DNA. Hum Mol Genet 1996;5:563-569.
- Huxley C, Passage E, Robertson AM, Youl B, Huston S, Manson A, Sabéran-Djoniedi D, Figarella-Branger D, Pellissier JF, Thomas PK, Fontés M. Correlation between varying levels of PMP22 expression and the degree of demyelination and reduction in nerve conduction velocity in transgenic mice. Hum Mol Genet 1998;7:449-458.
- Robertson AM, Huxley C, King RH, Thomas PK. Development of early postnatal peripheral nerve abnormalities in Trembler-J and PMP22 transgenic mice. J Anat 1999;195:331-339.
- Verhamme C, King RH, ten Asbroek AL, Muddle JR, Nourallah M, Wolterman R, Baas F, van Schaik IN. Myelin and axon pathology in a long-term study of PMP22-overexpressing mice. J Neuropathol Exp Neurol 2011;70:386-398.
- Chittoor VG, Sooyeon L, Rangaraju S, Nicks JR, Schmidt JT, Madorsky I, Narvaez DC, Notterpek L. Biochemical characterization of protein quality control mechanisms during disease progression in the C22 mouse model of CMT1A. ASN Neuro 2013;5:e00128.
- Ino D, Iino M.
In vivo gene transfer to Schwann cells in the rodent sciatic nerve by electroporation. J Vis Exp 2016;115:e54567. - Lee J, Jung SC, Joo J, Choi YR, Moon HW, Kwak G, Yeo HK, Lee JS, Ahn HJ, Jung N, Hwang S, Rheey J, Woo SY, Kim JY, Hong YB, Choi BO. Overexpression of mutant HSP27 causes axonal neuropathy in mice. J Biomed Sci 2015;22:43.
- Fledrich R, Stassart RM, Klink A, Rasch LM, Prukop T, Haag L, Czesnik D, Kungl T, Abdelaal TA, Keric N, Stadelmann C, Brück W, Nave KA, Sereda MW. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med 2014;20:1055-1061.
- Kaya F, Belin S, Bourgeois P, Micaleff J, Blin O, Fontés M. Ascorbic acid inhibits PMP22 expression by reducing cAMP levels. Neuromuscul Disord 2007;17:248-253.
- Sahenk Z, Nagaraja HN, McCracken BS, King WM, Freimer ML, Cedarbaum JM, Mendell JR. NT-3 promotes nerve regeneration and sensory improvement in CMT1A mouse models and in patients. Neurology 2005;65:681-689.
- Sahenk Z, Galloway G, Edwards C, Malik V, Kaspar BK, Eagle A, Yetter B, Forgie A, Tsao D, Lin JC. TrkB and TrkC agonist antibodies improve function, electrophysiologic and pathologic features in Trembler J mice. Exp Neurol 2010;224:495-506.
- Sahenk Z, Galloway G, Clark KR, Malik V, Rodino-Klapac LR, Kaspar BK, Chen L, Braganza C, Montgomery C, Mendell JR. AAV1.NT-3 gene therapy for Charcot-Marie-Tooth neuropathy. Mol Ther 2014;22:511-521.
- Attarian S, Vallat JM, Magy L, Funalot B, Gonnaud PM, Lacour A, Péréon Y, Dubourg O, Pouget J, Micallef J, Franques J, Lefebvre MN, Ghorab K, Al-Moussawi M, Tiffreau V, Preudhomme M, Magot A, Leclair-Visonneau L, Stojkovic T, Bossi L, Lehert P, Gilbert W, Bertrand V, Mandel J, Milet A, Hajj R, Boudiaf L, Scart-Grès C, Nabirotchkin S, Guedj M, Chumakov I, Cohen D. An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A. Orphanet J Rare Dis 2014;9:199.
- Chumakov I, Milet A, Cholet N, Primas G, Boucard A, Pereira Y, Graudens E, Mandel J, Laffaire J, Foucquier J, Glibert F, Bertrand V, Nave KA, Sereda MW, Vial E, Guedj M, Hajj R, Nabirotchkin S, Cohen D. Polytherapy with a combination of three repurposed drugs (PXT3003) down-regulates Pmp22 over-expression and improves myelination, axonal and functional parameters in models of CMT1A neuropathy. Orphanet J Rare Dis 2014;9:201.
- Zhao HT, Damle S, Ikeda-Lee K, Kuntz S, Li J, Mohan A, Kim A, Hung G, Scheideler MA, Scherer SS, Svaren J, Swayze EE, Kordasiewicz HB. PMP22 antisense oligonucleotides reverse Charcot-Marie-Tooth disease type 1A features in rodent models. J Clin Invest 2018;128:359-368.
- Lee JS, Chang EH, Koo OJ, Jwa DH, Mo WM, Kwak G, Moon HW, Park HT, Hong YB, Choi BO. Pmp22 mutant allele-specific siRNA alleviates demyelinating neuropathic phenotype in vivo. Neurobiol Dis 2017;100:99-107.
- Gokey NG, Srinivasan R, Lopez-Anido C, Krueger C, Svaren J. Developmental regulation of microRNA expression in Schwann cells. Mol Cell Biol 2012;32:558-568.
- Letzen BS, Liu C, Thakor NV, Gearhart JD, All AH, Kerr CL. MicroRNA expression profiling of oligodendrocyte differentiation from human embryonic stem cells. PLoS One 2010;5:e10480.
- Wang C, Ji B, Cheng B, Chen J, Bai B. Neuroprotection of microRNA in neurological disorders (Review). Biomed Rep 2014;2:611-619.
- Sontheimer EJ, Carthew RW. Silence from within: endogenous siRNAs and miRNAs. Cell 2005;122:9-12.
- Askou AL, Alsing S, Holmgaard A, Bek T, Corydon TJ. Dissecting microRNA dysregulation in age-related macular degeneration: new targets for eye gene therapy. Acta Ophthalmol 2018;96:9-23.
- Karakikes I, Chaanine AH, Kang S, Mukete BN, Jeong D, Zhang S, Hajjar RJ, Lebeche D. Therapeutic cardiactargeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc 2013;2:e000078.
- Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW. Systemic delivery of microRNA-181b inhibits nuclear factor-κB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res 2014;114:32-40.
- Heller KN, Mendell JT, Mendell JR, Rodino-Klapac LR. MicroRNA-29 overexpression by adeno-associated virus suppresses fibrosis and restores muscle function in combination with micro-dystrophin. JCI Insight 2017;2:e93309.
- Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer - an emerging concept. EBioMedicine 2016;12:34-42.
- Ji W, Sun B, Su C. Targeting microRNAs in cancer gene therapy. Genes (Basel) 2017;8:21.



