Necessity of electrically conductive pili for methanogenesis with magnetite stimulation
- Published
- Accepted
- Received
- Academic Editor
- Xiaomin Li
- Subject Areas
- Microbiology, Molecular Biology, Biogeochemistry
- Keywords
- Geobacter metallireducens, Methanosarcina barkeri, Electrically conductive pili (e-pili), Direct interspecies electron transfer (DIET), Magnetite, Ferrous iron, Ethanol metabolism, Stimulation, Co-cultures, Methane
- Copyright
- © 2018 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. Necessity of electrically conductive pili for methanogenesis with magnetite stimulation. PeerJ 6:e4541 https://doi.org/10.7717/peerj.4541
Abstract
Background
Magnetite-mediated direct interspecies electron transfer (DIET) between Geobacter and Methanosarcina species is increasingly being invoked to explain magnetite stimulation of methane production in anaerobic soils and sediments. Although magnetite-mediated DIET has been documented in defined co-cultures reducing fumarate or nitrate as the electron acceptor, the effects of magnetite have only been inferred in methanogenic systems.
Methods
Concentrations of methane and organic acid were analysed with a gas chromatograph and high-performance liquid chromatography, respectively. The concentration of HCl-extractable Fe(II) was determined by the ferrozine method. The association of the defined co-cultures of G. metallireducens and M. barkeri with magnetite was observed with transmission electron micrographs.
Results
Magnetite stimulated ethanol metabolism and methane production in defined co-cultures of G. metallireducens and M. barkeri; however, magnetite did not promote methane production in co-cultures initiated with a culture of G. metallireducens that could not produce electrically conductive pili (e-pili), unlike the conductive carbon materials that facilitate DIET in the absence of e-pili. Transmission electron microscopy revealed that G. metallireducens and M. barkeri were closely associated when magnetite was present, as previously observed in G. metallireducens/G. sulfurreducens co-cultures. These results show that magnetite can promote DIET between Geobacter and Methanosarcina species, but not as a substitute for e-pili, and probably functions to facilitate electron transfer from the e-pili to Methanosarcina.
Conclusion
In summary, the e-pili are necessary for the stimulation of not only G. metallireducens/G. sulfurreducens, but also methanogenic G. metallireducens/M. barkeri co-cultures with magnetite.
Introduction
Microbial methane production is one of the most successful, large-scale bioenergy strategies (Liu et al., 2009; Shen et al., 2016) and methane production in terrestrial environments is a major source of atmospheric methane, an important greenhouse gas (Bridgham et al., 2013; Conrad, 2007). In freshwater methanogenic environments, and anaerobic digesters, methanogens primarily produce methane from the metabolism of acetate and the reduction of carbon dioxide with H2 to methane. The well-known source of electrons for carbon dioxide reduction to methane is H2 (Sieber, McInerney & Gunsalus, 2012); however, it has recently been demonstrated that Methanosaeta and Methanosarcina species can accept electrons from the donor strain G. metallireducens for carbon dioxide reduction via direct interspecies electron transfer (DIET) (Chen et al., 2014a; Chen et al., 2014b; Rotaru et al., 2014a; Rotaru et al., 2014b; Wang et al., 2016).
In the absence of added conductive materials, DIET between Geobacter metallireducens and Methanosaeta and Methanosarcina species requires the electrically conductive pili (e-pili) of G. metallireducens (Chen et al., 2014a; Rotaru et al., 2014a; Rotaru et al., 2014b). The e-pili of both Geobacter species are also required for DIET in co-cultures of G. metallireducens and G. sulfurreducens (Shrestha et al., 2009; Summers et al., 2010). Existing studies on the e-pili of G. sulfurreducens have suggested that the conductivity along the length of Geobacter e-pili (Adhikari et al., 2016; Malvankar & Lovley, 2014) can be attributed to the tight packing of aromatic amino acids within the pilus structure, which confer a metallic-like conductivity similar to that observed in carbon nanotubes (Malvankar et al., 2015; Malvankar et al., 2011; Vargas et al., 2013). The e-pili are decorated with the c-type cytochrome OmcS, which does not contribute to conductivity along the length of the e-pili, but is important for electron transfer from the e-pili to extracellular electron acceptors/donors (Leang et al., 2010; Liu et al., 2015; Malvankar & Lovley, 2014; Malvankar, Tuominen & Lovley, 2012; Mehta et al., 2005; Summers et al., 2010). It is expected that the e-pili of G. metallireducens function in a similar manner (Smith, Lovley & Tremblay, 2013; Tremblay et al., 2012; Zheng et al., 2017), but the cytochrome(s) that are attached to the e-pili of G. metallireducens have not yet been identified.
Conductive carbon materials, such as: granular activated carbon, carbon cloth, and biochar, stimulate DIET (Chen et al., 2014a; Chen et al., 2014b; Liu et al., 2012; Rotaru et al., 2014a). The electron-donating and electron-accepting partners both attach to the conductive carbon materials, which serve as an electrical conduit between the two species. Mutant Geobacter strains that lack e-pili can participate in DIET under these conditions, presumably because biological cell-to-cell electrical conduits are no longer required (Chen et al., 2014a; Chen et al., 2014b; Liu et al., 2012; Rotaru et al., 2014a).
An important insight into carbon and electron flow in methanogenic environments lies in the finding that magnetite stimulated methane production in enrichment cultures inoculated from paddy soil with either ethanol or acetate as the electron donor (Kato, Hashimoto & Watanabe, 2012a). The enhanced methane production was accompanied by an enrichment of the Geobacter and Methanosarcina species (Kato, Hashimoto & Watanabe, 2012a). It was hypothesised that the magnetite provided electrical contact between the Geobacter and Methanosarcina species and that the Geobacter species oxidized the ethanol or acetate to carbon dioxide with electron transfer to the Methanosarcina, which then used the electrons to reduce carbon dioxide to methane (Kato, Hashimoto & Watanabe, 2012a). Many subsequent studies have documented the fact that magnetite accelerates methane production in samples from sediments or anaerobic digesters or defined co-cultures and have also inferred that this can be attributed to enhanced electron transfer through magnetite to methanogens (Li et al., 2015; Tang et al., 2016; Yang et al., 2015; Zhuang et al., 2015). Magnetite does promote interspecies electron transfer between Geobacter sulfurreducens and Thiobacillus denitrificans growing with acetate as the electron donor and nitrate as the electron acceptor (Kato, Hashimoto & Watanabe, 2012b), as well as between G. metallirducens and G. sulfurreducens growing with ethanol as the electron donor and fumarate as the electron acceptor (Liu et al., 2015), however, it has never been directly demonstrated that magnetite promotes DIET to methanogens. Analysis of the mechanisms by which magnetite enhanced DIET in G. metallireducens/G. sulfurreducens co-cultures indicated that, unlike conductive carbon materials, magnetite does not act as a substitute for e-pili, but rather can take the place of OmcS by attaching to e-pili to facilitate DIET, thus alleviating the need for OmcS production (Liu et al., 2015). Therefore, it should not be assumed that magnetite promotes DIET to methanogens as has been demonstrated for conductive carbon materials. The purpose of this study was to evaluate further the possibility that magnetite promotes DIET to methanogens.
Materials and Methods
Microorganisms, media, and growth conditions
Wild-type Geobacter metallireducens strain GS-15 (ATCC 53774) (Aklujkar et al., 2009; Lovley et al., 1993) and a strain of G. metallireducens in which the gene for PilA, the pilus monomer, was deleted (Tremblay et al., 2012) were obtained from our laboratory collection. Methanosarcina barkeri strain DSM 800 (ATCC 43569) was obtained from DSMZ (Braunschweig, Germany).
All culturing was performed under strict anaerobic conditions under a gas phase of N2/CO2 (80/20). Inocula for co-cultures were developed by growing G. metallireducens strains in Fe(III)-citrate (FC) medium (Bagnara et al., 1985), with 20 mmol L−1 ethanol as the sole electron donor and 55 mmol L−1 Fe(III) citrate as the electron acceptor. For co-cultures of G. metallireducens and M. barkeri, G. metallireducens was grown in DSMZ methanogenic medium 120 with 20 mmol L−1 ethanol as the electron donor and nitrate (10 mmol L−1) as the electron acceptor. M. barkeri was grown in the same medium with 30 mmol L−1 acetate as the substrate. Co-cultures were grown in 40 mL medium 120 with a 10% inoculum and with ethanol (20 mmol L−1) as the electron donor as described previously (Rotaru et al., 2014a). The incubation temperature for all methanogenic studies was 37 °C. When noted, magnetite was prepared as previously described (Kang et al., 1996) and added from stock solutions to give a final concentration of 5 mmol L−1 before autoclaving.
Chemical analysis
The gaseous samples were regularly collected from enrichment cultures with pressure-lock analytical syringes. The concentrations of CH4 were analysed with a gas chromatograph (GC-7890A; Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionisation detector.
Concentrations of ethanol and acetate were analysed with high-performance liquid chromatography (1260 Infinity; Agilent Technologies, Santa Clara, CA, USA) with a Hi-plex H column equipped with a refractive index detector.
The concentration of HCl-extractable Fe(II) was extracted from cultures and each replicate of the assays in triplicate as described previously (Zheng et al., 2015). Moreover, the concentration of dissolved Fe(II) in samples was also quantified by filtering through 0.45 µm sterile syringe filters and using the ferrozine method as described above.
Microscopy
Samples of cells and magnetite were negatively stained with 2% phosphotungstic acid and examined by a JEM-1400 (JEOL, Japan) transmission electron microscope (TEM).
Results and Discussion
Magnetite stimulation of DIET between G. metallireducens and M. barkeri
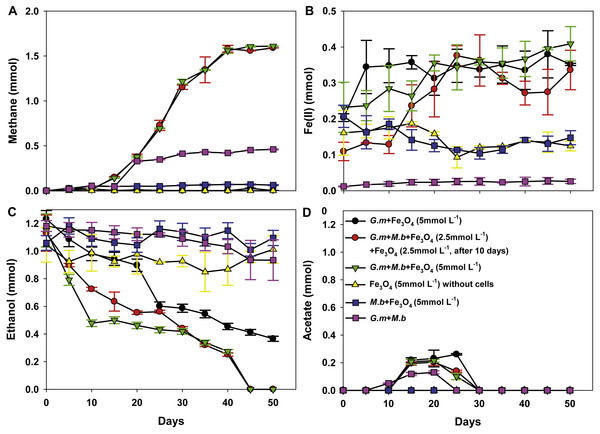
To evaluate whether, or not, magnetite was capable of stimulating DIET between G. metallireducens and M. barkeri, co-cultures were initiated with ethanol as the sole electron donor in the presence, and absence, of magnetite. Although M. bakeri is capable of using H2 as an electron donor, G. metallireducens cannot metabolise ethanol with the production of H2 (Rotaru et al., 2014b; Shrestha et al., 2013a; Summers et al., 2010) and thus syntrophic growth in G. metallireducens/M. barkeri co-cultures can be attributed to DIET. First, based on Fig. 1A, the production of CH4 from the co-culture of G. metallireducens and M. barkeri without magnetite indeed indicates DIET in syntrophic interaction. However, DIET is not exclusively occurred, at least a part of methane was still produced from acetate. For example, if half of the methane (∼0.25 mmol) was produced from acetate (∼0.25 mmol), the concentration of acetate remained in the medium should be ∼0.25 mmol.
Figure 1: Co-cultures of G. metallireducens (G. m) and M. barkeri (M. b) with ethanol as the substrate in the presence, or absence, of magnetite (Fe3O4).
Quantities of methane (A), ferrous iron (B), ethanol (C), and acetate (D) in cultures. Data are the means and standard deviation for triplicate cultures. In some instances the standard deviation was less than the size of the symbol.The initial establishment of G. metallireducens and M. barkeri co-cultures requires a long adaption period in the absence of added conductive materials (Rotaru et al., 2014a). As expected, ethanol was only slowly metabolised over 50 days without magnetite (Fig. 1C), however, in the presence of magnetite, ethanol was metabolised with the production of methane beginning within 10 days (Fig. 1C). Non-inoculated controls with magnetite showed no ethanol metabolism or methane production.
Limited acetate accumulated in the G. metallireducens with, or without, M. barkeri co-cultures in the presence of magnetite (C2H6O + H2O → C2H4O2 + 4H+ + 4e−, Oxidation of one ethanol will produce one acetate plus four electrons released (Rotaru et al., 2014a)), but was later consumed (Fig. 1D), which differed from co-cultures of G. metallireducens and M. barkeri in the absence of magnetite, suggesting that G. metallireducens metabolised the acetate that G. metallireducens produced from ethanol compared with the result of G. metallireducens acting alone with magnetite. The high amount of methane in the G. metallireducens and M. barkeri co-cultures suggested that M. barkeri only used the electrons released from ethanol oxidation for reducing carbon dioxide to produce methane in the magnetite-amended co-cultures (8H+ + 8e− + CO2 → CH4 + 2H2O). The total amount of ethanol from the magnetite-amended co-cultures metabolised (1.15 ± 0.12 mmol) resulted in 1.60 ± 0.0032 mmol methane (Figs. 1A, 1C), which showed that the mmol ratio of CH4/C2H6O (1.60/1.15) was 1.39 (>1), thus about 92.2% of the electrons from ethanol oxidation were recovered in methane according to the equation: 2C2H5OH → 3CH4 + CO2. Furthermore, no H2 was detected in any of the experiment groups. This result was consistent with the fact that G. metallireducens is unable to produce H2 during ethanol metabolism (Shrestha et al., 2013b). Therefore, the high electron recovery that was available from ethanol to methane suggested that magnetite can stimulate DIET between G. metallireducens and M. barkeri and suggested that the simplest explanation for the enrichment of Geobacter and Methanosarcina observed in the presence of magnetite in previous studies (Kato, Hashimoto & Watanabe, 2012a) is that magnetite was facilitating DIET.
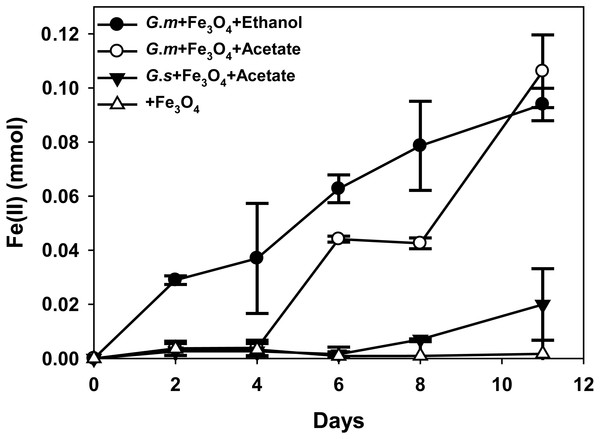
Figure 2: Quantities of ferrous iron in cultures of Geobacter metallireducens (G.m) and Geobacter sulfurreducens (G.s) in the presence of magnetite with ethanol and acetate as the substrates.
HCl-extractable ferrous iron was also produced in G. metallireducens-M. barkeri co-cultures from reduction ferric iron of magnetite within five days and increased to 0.1768 ± 0.0219 mmol at 50 days, which was equal to that when G. metallireducens was tested with magnetite alone (0.1761 ± 0.0549 mmol) (Fig. 1B); however, the concentration of dissolved ferrous iron was under detect limitation during the incubation of the co-cultures amended with magnetite, suggested that only a part of the ferric iron in the added magnetite was reduced to ferrous iron. The results indicated that only a small portion of electrons (about 4.6 mmol electrons released from 1.15 mmol ethanol oxidation, 0.18 mmol/4.6 mmol, about 3.9%) in G. metallireducens-M. barkeri co-cultures with magnetite were used for ferric iron reduction and the majority of electrons (about 96.1%) were used for methane production. This result differs from that reporting that magnetite acts as the electrical conduit between electron-donating Geobacter and electron-accepting methanogens (Kato, Hashimoto & Watanabe, 2012a; Li et al., 2015; Viggi et al., 2014). One factor controlling ferrous iron production in co-cultures amended with magnetite is the range of substrates that can be metabolised by Geobacter species. G. metallireducens can utilise ethanol and acetate, ferrous iron production from acetate was slower than that from ethanol within 10 days in the presence of magnetite, while ferrous iron production from G. sulfurreducens amended with magnetite was much lower than that from G. metallireducens when utilising acetate (Fig. 2). This suggested that G. metallireducens, like some microorganisms (e.g., Shewanella, Dechloromonas, Desulfovibrio, and Clostridium) was able to use magnetite as the electron acceptor from ethanol or acetate metabolism (Kostka & Nealson, 1995; Yang et al., 2015). However, it is not possible for magnetite to act as the electron shuttle for production of methane from carbon dioxide because of the relatively high mid-point potential of the Fe(III)/Fe(II) redox couple (E 0’ = +0.20 V, pH 7.0) which is too high for the reduction of carbon dioxide to methane (E 0’ of CO2/methane couple = −240 mV).
To determine the actual role of magnetite in stimulation of ethanol metabolism and methane production in co-cultures of wild-type G. metallireducens and M. barkeri, co-cultures were initiated with 2.5 mmol L−1 magnetite, after a 10-day incubation period, an additional 2.5 mmol L−1 magnetite was subsequently added. Methane production presented the same tendency with 5 mmol L−1 magnetite added to the co-cultures (Fig. 1A): this meant that the manner and amount of addition of magnetite could not affect methane production, however, the amount of HCl-extractable ferrous iron changed: the reduced ferrous iron concentration was about 0.0193–0.0239 mmol (∼9.6–12% of added Fe3 +) when 2.5 mmol L−1 magnetite (Fe3 +: 0.2 mmol) was added during the first 10 days, and subsequently reduced when more magnetite was added, the amount of ferrous iron used in each step (total: 0.1635 ± 0.0313 mmol) was similar to the addition of 5 mmol L−1 magnetite (Fig. 1B). This result was consistent with the observation that G. metallireducens alone reduced magnetite to produce ferrous iron (Fig. 1B). Thus, the initial concentration of magnetite determined how much Fe(III) inside was reduced. When high concentration of magnetite (5 mmol L−1) was available, Fe(III) reduction was detected; however, no significant Fe(III) reduction was found when lower concentration of magnetite (2.5 mmol L−1) was present. Fe(III) in the magnetite was reduced only when additional magnetite (2.5 mmol L−1) was added. This demonstrated that lower concentration of magnetite could not be preferentially used as the electron acceptor in the co-culture of G. metallireducens and M. barkeri. Similarly, ethanol was stimulated to oxidise and little acetate was transiently accumulated in magnetite upon its step-by-step addition to co-cultures of wild-type G. metallireducens and M. barkeri (Figs. 1C, 1D). The calculation of electron recovery (93.81%) of electrons available from ethanol in methane in these samples further suggested that M. barkeri was accepting electrons from carbon dioxide reduction via DIET.
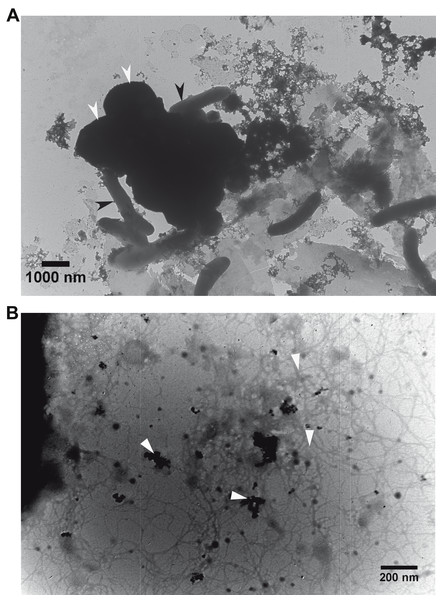
Transmission electron microscopy (TEM) revealed that G. metallireducens (rod-shaped cells) and M. barkeri (larger size cocci) were associated with each other (Fig. 3A). With higher magnification it was apparent that magnetite was associated with the G. metallireducens pili (Fig. 3B), as was previously observed that some of the magnetite was localised along pili and compensated for the lack of OmcS of G. sulfurreducens in promoting electrical contacts with pili in G. metallireducens/G. sulfurreducens co-cultures (Liu et al., 2015).
Figure 3: Transmission electron micrographs.
Association of the defined co-cultures of G. metallireducens and M. barkeri with magnetite. (A) Association of the two cell types. Black and white arrows indicate G. metallireducens cells and M. barkeri cells, respectively. (B) Association of magnetite with pili. White arrows indicate magnetite and pili.Failure of magnetite to compensate for loss of e-pili in G. metallireducens
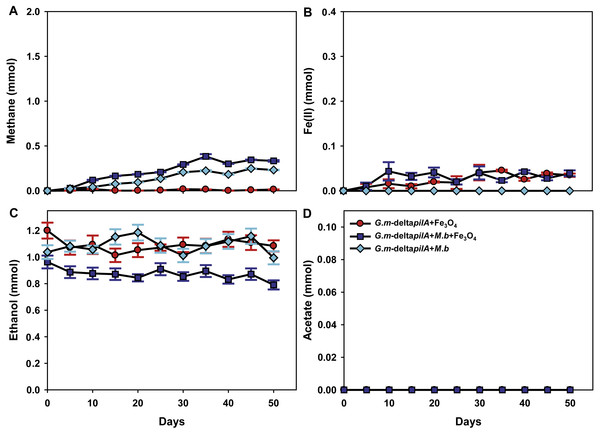
To investigate further the mechanisms for magnetite stimulation of DIET between G. metallireducens and M. barkeri, co-cultures were initiated with the previously described strain of G. metallireducens (Tremblay et al., 2012) that is incapable of producing pili because the gene for PilA, the pilus monomer, has been deleted. As expected from previous studies (Rotaru et al., 2014a), methane was not produced in co-cultures with the pili-deficient strain of G. metallireducens (Fig. 4A); however, co-cultures amended with magnetite produced less methane (about 0.38 ± 0.025 mmol, Fig. 4A). During co-culture testing, ferrous iron concentrations were below 0.1 mmol in magnetite amended cultures (Fig. 4B). Furthermore, co-cultures with the pil A-deficient G. metallireducens failed to metabolise ethanol or produce acetate with, or without, magnetite amendment (Figs. 4C, 4D). These results suggested that magnetite perhaps can partly substitute for pili to participate in DIET of co-cultures resulting from its electrical conductivity; however, magnetite appears to promote DIET by a mechanism that is different than that in conductive carbon materials such as GAC and carbon cloth (Chen et al., 2014a; Liu et al., 2012). In the presence of GAC or carbon cloth the pili-deficient strain of G. metallireducens can transfer electrons to M. barkeri because both species attach to the conductive materials, which are much bigger than individual cells. Magnetite particles are typically smaller (at 20–50 nm) than cells and thus are unlikely to provide effective cell-to-cell contacts (Liu et al., 2015). This was evident in previous studies with G. metallireducens/G. sulfurreducens co-cultures in which magnetite was not able to compensate for the lack of e-pili in G. metallireducens (Liu et al., 2015). Multiple lines of evidence, including studies with an OmcS-deficient mutant, suggested that magnetite could serve as the functional equivalent of OmcS, and the c-type cytochrome associated with the e-pili of G. sulfurreducens (Liu et al., 2015). Similar genetic experiments are not yet possible with G. metallireducens because the cytochrome(s) associated with the G. meatllireducens e-pili have not been identified. However, the finding that magnetite amendments did not permit the growth of the pili-deficient strain of G. metallireducens in co-culture with M. barkeri, suggested the magnetite cannot function as an e-pili substitute in all regards. Magnetite was associated with the e-pili in the G. metallireducens/M. barkeri co-cultures. Therefore, it is likely that magnetite also facilitated electron transfer from the G. metallireducens e-pili to M. barkeri in the co-cultures.
Figure 4: Co-cultures of M. barkeri (M. b) and a PilA-deficient G.metallireducens (G. m-deltapilA) strain in the presence, or absence, of magnetite (Fe3O4).
Quantities of methane (A), ferrous iron (B), ethanol (C), and acetate (D) in cultures. Data are the means and standard deviation for triplicate cultures. In some instances the standard deviation was less than the size of the symbol.Conclusions
In sum, we have established co-cultures of M. barkeri and wild-type G. metallireducens or a strain deficient in the PilA gene with or without magnetite. The results revealed magnetite stimulated ethanol metabolism and methane production in defined co-cultures of G. metallireducens and M. barkeri. However, magnetite did not promote methane production in co-cultures of the pilA-deficient G. metallireducens. These results showed that magnetite could not substitute for e-pili to promote DIET between Geobacter and Methanosarcina species, in which the e-pili are necessary for the stimulation.