-
PDF
- Split View
-
Views
-
Cite
Cite
Ajay Narendra, J Frances Kamhi, Yuri Ogawa, Moving in Dim Light: Behavioral and Visual Adaptations in Nocturnal Ants, Integrative and Comparative Biology, Volume 57, Issue 5, November 2017, Pages 1104–1116, https://doi.org/10.1093/icb/icx096
Close - Share Icon Share
Synopsis
Visual navigation is a benchmark information processing task that can be used to identify the consequence of being active in dim-light environments. Visual navigational information that animals use during the day includes celestial cues such as the sun or the pattern of polarized skylight and terrestrial cues such as the entire panorama, canopy pattern, or significant salient features in the landscape. At night, some of these navigational cues are either unavailable or are significantly dimmer or less conspicuous than during the day. Even under these circumstances, animals navigate between locations of importance. Ants are a tractable system for studying navigation during day and night because the fine scale movement of individual animals can be recorded in high spatial and temporal detail. Ant species range from being strictly diurnal, crepuscular, and nocturnal. In addition, a number of species have the ability to change from a day- to a night-active lifestyle owing to environmental demands. Ants also offer an opportunity to identify the evolution of sensory structures for discrete temporal niches not only between species but also within a single species. Their unique caste system with an exclusive pedestrian mode of locomotion in workers and an exclusive life on the wing in males allows us to disentangle sensory adaptations that cater for different lifestyles. In this article, we review the visual navigational abilities of nocturnal ants and identify the optical and physiological adaptations they have evolved for being efficient visual navigators in dim-light.
Introduction
Efficient navigation is crucial for most daily tasks including foraging, finding mates, establishing territories, and parental care. While the scale of navigation varies between animals (from a few centimeters to several kilometers), the principles of navigation are surprisingly similar (Biro et al. 2007; Wehner 2009). Even what appears to be a relatively simple task of walking in a straight line cannot be achieved without an external visual compass (Cheung et al. 2007). Ants, despite their tiny size, relatively small brains and few neurons, are highly competent visual navigators. Several ant species restrict their activity to brightly lit periods during the day where visual information is reliable. A significant number of ants, however, are active in dimly lit environments that include animals that forage in the dark confines of the leaf-litter, in closed canopy rain forests, or at night. In dim-light habitats, the visual signal-to-noise ratio is typically low, which makes detecting reliable visual navigational information a challenge (Warrant et al. 2004; Warrant 2017). This is especially true at night, where light intensities can be 11 orders of magnitude lower than during the day (O’Carroll and Warrant 2017). We will here review the behavioral, optical, and physiological adaptations that ants have evolved for being efficient visual navigators in dim-light.
Visually guided behavior in nocturnal ants
A time to forage and a time to fly
An unusual aspect of ant sensory ecology is that each caste in an ant society performs a unique set of visual tasks despite their similar genotypes. Workers are typically sterile females and exclusively pedestrian and carry out all the day-to-day foraging and above-ground nest maintenance activities that require vision. Alates are the reproductive winged castes of females and males. During the first stage of their life, winged females require vision: they fly out of the nest for mating, following which they shed their wings, and become pedestrian. They then start a new nest and remain in it for the rest of their lives within the dark confines of the nest chambers where vision plays no role. Males exclusively engage in mating, which involves flying out of the nest to mate, visually tracking flying females and competitors and carrying out aerial pursuits.
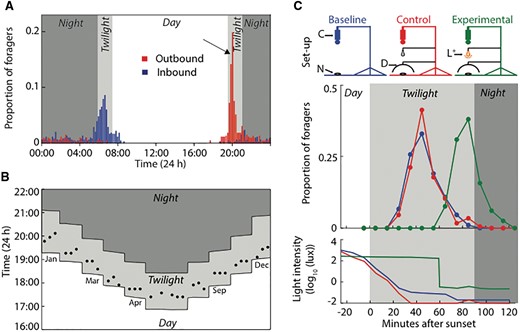
The earliest evidence that ants react to light came from John Lubbock who exposed the brood of Lasius flavus and Formica fusca to ultraviolet light (Lubbock 1882). Ants responded by moving their brood to regions beyond the red wavelength. Following this, the effect of light intensity on ants remained less explored, perhaps because surface temperature and humidity were considered the main abiotic factors to regulate ant activity (Eidmann 1935). The focus shifted back to the importance of light cues when it was first demonstrated that ambient light intensity could trigger foraging activity in the day-active leafcutter ants Atta colombica (Hodgson 1955). Edward Hodgson observed that foraging began earlier on trails that received light first and later on trails that were in the shade. Increasing the light intensity around sunrise caused the animals to become active earlier than under natural conditions and decreasing the light intensity caused a delay in the foraging onset. But increasing the light intensity after the last forager had returned did not trigger activity, indicating that diurnal ants responded to early availability of light only when it occurred close to their natural foraging time. Ambient light intensity also dictates the time at which nocturnal ants depart from the nest. In the nocturnal bull ant Myrmecia pyriformis, workers typically begin foraging just after sunset and restrict their nest departures to the evening twilight (Fig. 1A, B) (Narendra et al. 2010). When light intensity around sunset was artificially increased, ants delayed their exit and began foraging only when light intensity was decreased to correspond to the typical ambient evening twilight condition (Fig. 1C). The endogenous rhythm likely brings animals close to the nest entrance, but exiting the nest appears to be controlled by ambient light intensity. Further evidence that light influences ant activity comes from investigation into circadian rhythms. For instance, diurnal workers of Formica polyctena that were entrained to alternating light and dark cycles increased their activity toward the end of the dark period as if anticipating dawn and the onset of foraging (Rosengren 1977, 1986). Monitoring ambient light to trigger activity is crucial for alates since they must synchronize the timing of mating flights between nests to mate at species-specific times (McCluskey 1965, 1992).
Ambient light intensity triggers foraging in a nocturnal ant, Myrmecia pyriformis. (A) Daily activity schedule on a single summer day shows that a majority of the individuals leave the nest in the evening twilight and return in the morning twilight. Arrow indicates the peak foraging in the evening twilight. (B) Peak foraging activity occurs strictly during the evening twilight over the entire year. The time of peak outbound activity (indicated by an arrow in 1A) was determined by monitoring activity pattern at a single nest throughout the year. Observations were done in blocks of three consecutive days and repeated at every 30-min change in sunset time throughout the year. Each filled circle refers to the peak-activity time on a single day. There was no activity on one day in May when surface temperatures at sunset was <7 °C. (C) Ambient light intensity triggers the foraging onset. Foraging activity above the nest and ambient light intensity was filmed on three different days. Baseline: activity was filmed with a camera (C) from above the nest (N); Control: activity was filmed with a diffuser (D) placed above the nest; Experimental: activity was filmed with a light source (L) ON for 60 min after sunset and then switched OFF. Activity and light intensity in the three conditions are shown. Figures modified from Narendra et al. (2010).
Knowing where to go
Ants are traditionally thought to navigate primarily using pheromone trails. Trail pheromones are indeed utilized by several species but typically for recruitment. Even among trail-following species visual information is used by scouts to locate a food source and by experienced individuals that ignore pheromone trails to reach their goal faster (e.g., Hölldobler 1974; Card et al. 2016). To reach a goal of interest, ants require a compass to maintain a heading direction, a strategy to pinpoint a goal once they are close to it, an estimate of distance traveled and a knowledge of where they are. Day-active ants are known to use both celestial and terrestrial cues to accomplish these tasks (e.g., Wehner 2009). Here we will discuss the visual navigational strategies used by nocturnal ants.
Celestial cues
The earliest record of the use of visual information for nocturnal navigation in ants comes from Santchi’s observation that Monomorium salomonis ants walking in a straight line at night get disoriented when the clouds cover the moon (Santschi 1911; Papi 1960). The role of the moon in providing compass information has been investigated in F. polyctena (Jander 1957), Formica rufa (Kaul and Kopteva 1982), and Camponotus pennsylvanicus (Klotz and Reid 1993). Ants reversed their foraging direction when the position of the moon was mirrored, demonstrating that they can derive compass information from the moon. On nights with bright moonlight (350–5000 lux), even the strictly day-active desert ant, Cataglyphis bicolor, relied on the moon to obtain compass information (Wehner and Duelli 1971; Duelli 1972). In the strictly nocturnal M. pyriformis, a greater proportion of outbound and inbound activity occurred on full-moon nights when the lunar illumination was 95% compared with that on moonless nights when the lunar illumination was 0% (Reid et al. 2013). Thus, lunar illumination can enhance the salience of navigational cues in dim-light, leading to increased activity.
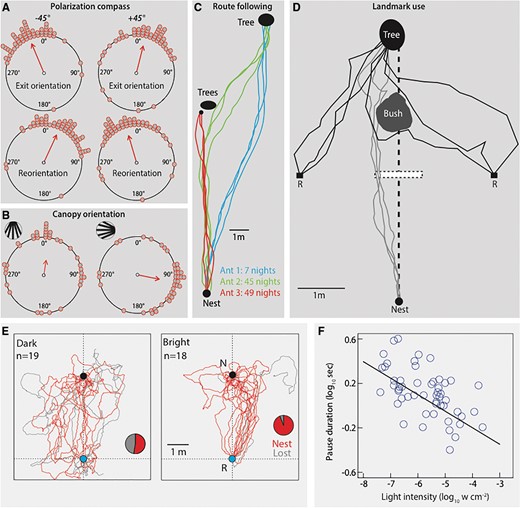
Similar to day-active ants (Wehner and Wehner 2001), the nocturnal ant M. pyriformis derives compass information from the pattern of polarized skylight (Reid et al. 2011). This has been demonstrated by rotating a polarized filter by +45° or −45° relative to the ambient orientation above the ant that is heading toward a particular goal (Fig. 2A). Ants that encountered such a change in the polarization pattern modified their orientation according to the rotation of the polarized filter. Upon exiting, they encountered the ambient polarized skylight and reoriented appropriately. Although the exit-orientation of ants was in the expected direction, the extent to which they changed their orientation was weak. The reason for this has become clear from another nocturnal species, Myrmecia midas (Freas et al. 2017b). The extent to which individual ants rely on the pattern of polarized skylight is dependent on the distance traveled and on their foraging state (i.e., outbound: to find food; inbound: returning to the nest). Inbound ants responded more to the change in the orientation of the polarization pattern compared with the outbound ants (Freas et al. 2017b). In addition, the greater the distance the animals traveled, the stronger their response to a change in the pattern of the polarized skylight (Freas et al. 2017b). This variation in the magnitude of response is most likely because Myrmecia ants rely predominantly on terrestrial visual cues for navigation (Narendra and Ramirez-Esquivel 2017).
Navigational strategies used by nocturnal ants. (A) Nocturnal M. pyriformis foragers derive compass information from the pattern of polarized skylight. A polarization filter placed above a foraging ant was rotated either −45° (left column) or + 45° (right column) relative to the ambient pattern of polarized skylight. Ants respond predictably by exiting the polarization filter in the appropriate direction (Exit orientations) and reorienting when they experienced the ambient skylight polarization pattern (Reorientation). Data for each circular plot are pooled from two nests and modified from Reid et al. (2011). (B) The trap jaw ant Odontomachus hastatus obtains compass information from the canopy pattern. Ants trained to a striped canopy pattern (left inset) were well oriented in the control condition (left). When the striped pattern was rotated by 90° (right), ants also modified their heading direction accordingly. Mean direction and length of the mean vector are indicated by an arrow in each circular plot. Figure modified with permission from Rodrigues and Oliveira (2014). (C) Idiosyncratic routes of three nocturnal ants, M. pyriformis over 7 nights, 45 nights, and 49 nights showing fidelity toward route and foraging trees (Reid 2010). (D) Use of landmarks by a nocturnal ant, M. pyriformis. Typical foraging paths from nest to the tree are shown. Six ants were captured at the halfway mark (white rectangular box) and displaced by 2m either toward the left or right of the nest-tree line. Displaced ants either orient immediately toward the tree or head toward a conspicuous bush and detour around it to reach the tree. Adapted from Reid et al. (2011). (E) Homing efficiency of nocturnal M. pyriformis decreases at low light levels. Homing paths of ants released at the base of the foraging tree (R, blue circle) as they head toward the nest (N, black circle) in dark (60–120 min before sunrise) and bright (60–120 min after sunrise) light conditions. Pie graphs show the proportion of ants that reached the nest within the recording duration of 50 min. Nest: ants that successfully returned to the nest; Lost: ants that did not return to the nest. (F) The nocturnal worker ant M. pyriformis pause for longer durations as light level drops, a behavioral strategy to improve photon capture in low light. Modified from Narendra et al. (2013d).
Terrestrial cues
Similar to their day-active relatives, nocturnal ants rely on terrestrial landmarks in their dim-light environment to obtain compass information, to establish routes, and to pinpoint goals. The first evidence that ants orient using visual landmark information came from the trail-following ant L. flavus (Lubbock 1882). Ants trained to travel toward a distant candle changed their heading direction by 180° when rotated on a turntable by the same degree. One of the landmark features that ants use to obtain compass information is the overhead canopy pattern. For the short excursions that ants carry out, canopy patterns detected through the UV–green gradient can generate sufficient contrast in dim-light to provide directional information (Warrant and Dacke 2010). Canopy orientation in ants was first demonstrated in a day-active ant Paltothyreus tarsatus that operates in forested habitats where it has little access to direct sun or polarized skylight (Hölldobler 1980). It is only recently that canopy orientation has been demonstrated in a crepuscular–nocturnal ant, Odontomachus hastatus (Rodrigues and Oliveira 2014). Ants were trained to find food in an arena with a striped pattern as the roof. Ants tested with the canopy pattern rotated by 90° changed their orientation accordingly, demonstrating that ants derive compass information from the canopy pattern (Fig. 2B). Even trail-following ants such as the nocturnal C. pennsylvanicus orient using the canopy pattern (Klotz and Reid 1993). At present, it is unknown how and when ants learn the canopy pattern and whether different moonlight intensities affect the use of this information.
Nocturnal M. pyriformis ants use terrestrial landmarks to establish individualistic routes to specific Eucalyptus trees in their dimly lit habitats (Fig. 2C). They adhere to these routes for several weeks (Reid 2010). Slight modifications to the landmark panorama (removal of three dead trees) appear to create a significant mismatch to the ants between their previously learnt views and their current retinotopic view, disrupting their typical navigational behavior (Narendra and Ramirez-Esquivel 2017). Image difference analysis revealed very little difference between the before and after tree removal scenes. However, on the first encounter of the modified scene, the proportion of ants foraging reduced dramatically, and most individuals stayed close to the nest carrying out re-learning walks before heading out to forage on subsequent nights. It remains to be determined whether ants extract specific features from the landmark panorama or use the information from the entire panorama.
Path integration
Path integration is the ability to integrate distances traveled and angles steered on the outbound journey to compute the shortest vector home, a strategy that is well known in several day-active ant species (Müller and Wehner 1988; Beugnon et al. 2005; Narendra 2007; Narendra et al. 2013b, 2013c). Among nocturnal ants the ability to path integrate is known only in the genus Myrmecia. Foragers of both M. midas and M. pyriformis that had a home vector >10 m when displaced to unfamiliar locations relied on their path integrator. These ants typically traveled for a short distance before beginning a search (Narendra et al. 2013d; Freas et al. 2017a). Perhaps, ants travel for a short distance relying on the path integrator because they detect sufficient mismatch in the visual scene between the familiar and the displacement site, a behavior often seen in ants that occupy landmark rich habitats (e.g., Cheung et al. 2012).
Navigational knowledge
Very few studies have attempted to map the navigational knowledge of night-active ants. Such a demonstration has been done in the day-active ants by capturing animals returning home close to their nest and displacing them to locations where they have never been. In such experiments, the diurnal desert ant Cataglyphis fortis searches extensively around the release location and then heads directly home (Wehner et al. 1996). After being released in an unfamiliar location, another diurnal ant Myrmecia croslandi briefly scans the world and heads directly home without engaging in a search (Narendra et al. 2013b). In the nocturnal M. pyriformis, ants leaving the nest were captured about half-way from their food source and displaced by 2m toward the left or the right of the nest-tree line (Fig. 2D) (Reid et al. 2011). A majority of the displaced ants headed directly to the tree, while some detoured around a familiar bush and then headed to the tree. Even during homing, M. pyriformis ants captured at a food source and displaced to a location 12m lateral to their foraging corridor were able to return home (Narendra et al. 2013d). Thus, nocturnal ants also can reach a goal from locations they have not previously visited. It is unlikely that ants use a map (Cruse and Wehner 2011; Cheung et al. 2014), but they must have a familiar catchment zone within which they can find a goal through well-directed paths from novel locations. The extent of this catchment zone is likely to be dictated by the area covered during the learning walks and the landmark density in the habitat (Narendra et al. 2013b).
Navigational competence in dim-light
It is clear that night-active species rely on navigational strategies similar to their diurnal counterparts. However, are nocturnal navigators as efficient as their diurnal relatives? There is growing evidence to suggest that navigating at night is less efficient than during the day. The best evidence for this comes from comparing the navigational competence of a nocturnal ant species at different light intensities (Narendra et al. 2013d). Ants heading home in the dark at least an hour before sunrise had the least straight paths, the lowest success rate in finding home and the longest homing duration compared to ants returning home in bright conditions an hour after sunrise (Fig. 2E). Further, in the dark condition, homing ants that reached the nest vicinity searched longer for the nest entrance, indicating that pinpointing goals in dim-light may be difficult for ants. These differences in navigational efficiency between the bright and dark conditions suggest that pheromones may not aide in either route navigation or in pinpointing the nest.
Behavioral evidence suggests that accessing visual navigational information in the dark is also difficult. This is evident from experiments carried out on nocturnal M. pyriformis, where ants were captured at a food source and displaced to locations near the nest (∼12m). Ants displaced in bright conditions just before sunset were able to find home by well-directed paths, however, a majority of the ants displaced after sunset in the dark searched close to the release location. Accessing terrestrial visual information is also difficult when ants cannot maintain a stable head position. To match their current views to previously memorized views, maintaining a stable head position is critical. Head movements in ants around both the roll (Raderschall et al. 2016) and pitch axes (Ardin et al. 2015) can adversely affect visual navigation. Substantial changes in the head pitch or head roll occur when individual ants carry large pieces of food items or when they walk on uneven surfaces. Nocturnal ants compensated for 30–100% of their body roll when forced to walk on a twisted band (Raderschall et al. 2016). However, their ability to compensate for body roll deteriorated as light levels dropped, demonstrating that ants use visual information to stabilize head roll. It appears that the inefficiency of navigating at night and the difficulty of accessing reliable visual information has led to the nocturnal Myrmecia restricting their navigational tasks to the twilight period. To navigate efficiently in the dimly lit twilight periods, nocturnal ants have evolved a behavioral strategy to improve photon capture: they pause frequently and for longer durations as light levels drop (Fig. 2F) (Narendra et al. 2013d). This adaptation allows the eye to capture a brighter view of the world by increasing integration periods. Such behavioral adaptations are seen not only in nocturnal ants (Fourcassié et al. 1999; Narendra et al. 2013d), but also in bumblebees (Reber et al. 2015) and spiders (Nørgaard et al. 2008).
Optical adaptations for dim-light conditions
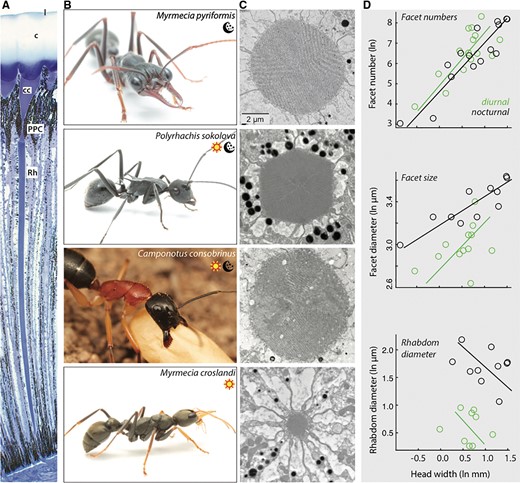
With few exceptions, ants have a pair of apposition compound eyes (Fig. 3), which is an eye design typical for insects active in bright light conditions. In an apposition eye, each eye contains several ommatidial units, and each ommatidium has its own lens, which captures light, and a crystalline cone that funnels light onto a photosensitive structure called a rhabdom (Fig. 3A). Each rhabdom is made up of microvilli from 8 to 9 retinula cells and contains the pigment rhodopsin that absorbs light and converts it into electrical neural signal (Warrant and Dacke, 2010; Tierney et al. 2017; Warrant 2017). Screening pigments ensheath each ommatidium, which ensures light travels within each ommatidium and is not shared between neighboring ommatidia (e.g., Narendra et al. 2016a). This organization limits the optical sensitivity of the eye. The “truly” nocturnal insects such as moths have superposition eyes where each rhabdom captures light from multiple facets, increasing their optical sensitivity (Land 1981). Nevertheless, ants active in dim-light conditions are competent visual navigators and it is hence essential to identify the optical and physiological adaptations they have evolved, which we discuss below.
The eyes of nocturnal ants. (A) Longitudinal section of the ommatidium of M. pyriformis showing lens (l), cornea (c), crystalline cone (cc), primary pigment cell (ppc), and rhabdom (rh). (B) Illustration of an exclusively nocturnal ant (top row), ants active during both day and night (second and third row) and an exclusively diurnal ant (bottom row). (C) Cross-section of rhabdoms as a transmission electron micrograph of species in (B). Scale bar for all cross-sections is shown in top panel. (D) Scatter plots of the best-fit standardized major axis regression lines of the relationship between head width and facet number (top), facet diameter (middle), and rhabdom diameter (bottom) in diurnal and nocturnal ants. Data compiled from Bernstein and Finn (1971); Menzel and Blakers (1975); Menzi (1987); Klotz et al. (1992); Baker and Ma (2006); Greiner at al. (2007); Gronenberg (2008); Narendra et al. (2011); Schwarz et al. (2011); Ramirez-Esquivel (2012); Narendra et al. (2013a); A. Narendra, unpublished data, and R. Nettimi, unpublished data). See also Table 1.
Eye design of a nocturnal ant
where A = largest facet diameter (µm); d = diameter of the rhabdom (µm); f = focal length, determined by the distance from the center of curvature of the inner corneal lens surface (as an estimate for the position of the nodal point) to the tip of the rhabdom; l = the rhabdom length; k = absorption coefficient assumed to be 0.0067 µm−1.
Summary of known facet numbers, facet diameters, and rhadbom diameters of ant species relative to their head width and activity time
| Species . | Head width (mm) . | Facet number . | Facet size (µm) . | Rhabdom diameter (µm) . | Activity . | References . |
|---|---|---|---|---|---|---|
| Camponotus consobrinus (major worker) | 3.1 | 798 | 34 | 7.8 | Nocturnal | Narendra et al. (2016b) |
| Camponotus detritus | 3.79 | 1300 | — | — | Diurnal | Menzi (1987) |
| Camponotus irritans | 2.11 | 350 | Nocturnal | Menzi (1987) | ||
| Camponotus ligniperda | 2.48 | 450 | — | — | Nocturnal | Menzi (1987) |
| Camponotus pennsylvanicus (minor worker) | 1.2 | 375 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus pennsylvanicus (major worker) | 3.5 | 658 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus sericeiventris (major worker) | 2.3 | 660 | — | — | Diurnal | R. Nettimi (2017, personal communication) |
| Cataglyphis bicolor (major worker) | 2.1 | 1300 | 24 | 2.5 | Diurnal | Menzi (1987) |
| Formica integroides | 1.9 | 680 | 19.3 | Diurnal | Bernstein and Finn (1971) | |
| Formica polyctena | 1.56 | 750 | 18.4 | 2.6 | Diurnal | Menzel and Blakers (1975) |
| Gigantiops destructor | 2.52 | 4100 | Diurnal | Gronenberg (2008) | ||
| Harpegnathos saltator | 2.2 | 1600 | 30 | 2.2 | Diurnal | A. Narendra (2016, personal observation) |
| Iridomyrmex purpureus | 2 | 496 | 14 | 2.3 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus bagoti (major worker) | 3.2 | 590 | 19 | 1.6 | Diurnal | Schwarz et al. (2011) |
| Melophorus hirsutus (major worker) | 1.7 | 296 | 19 | 1.4 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus hirsutus (minor worker) | 0.98 | 241 | 18 | 1.7 | Diurnal | Ramirez-Esquivel (2012) |
| Myrmecia croslandi | 2.04 | 2363 | 22 | 1.3 | Diurnal | Narendra et al. (2011) |
| Myrmecia desertorum (major worker) | 4.4 | 3625 | 38 | 5.8 | Nocturnal | A. Narendra (2016, personal observation) |
| Myrmecia nigriceps (major worker) | 3.6 | 3210 | 32.9 | 5.5 | Nocturnal | Narendra et al. (2011) |
| Myrmecia piliventris | 1.9 | 2288 | 22 | 1.3 | Diurnal | A. Narendra (2016, personal observation) |
| Myrmecia pyriformis (major worker) | 4.45 | 3593 | 37.5 | 5.9 | Nocturnal | Narendra et al. (2011) |
| Myrmecia pyriformis (small worker) | 2.3 | 2320 | 26 | 5.1 | Nocturnal | Greiner et al. (2007) |
| Myrmecia tarsata (major worker) | 3.7 | 2724 | 28.7 | 2.9 | Nocturnal | Narendra et al. (2011) |
| Nothomyrmecia macrops | 1.6 | 670 | 24.5 | 8.9 | Nocturnal | A. Narendra (2016, personal observation) |
| Notoncus ectatommoides | 1.3 | 209 | 26 | 5.9 | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (major worker) | 0.87 | 27 | 26 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (minor worker) | 0.42 | 21 | 20 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Polyrhachis sokolova | 1.93 | 596 | 33 | 5.0 | Nocturnal | Narendra et al. (2013a) |
| Solenopsis invicta (major worker) | 1.15 | 92.65 | 20.1 | — | Diurnal | Baker and Ma (2006) |
| Solenopsis invicta (minor worker) | 0.58 | 48 | 15.7 | — | Diurnal | Baker and Ma (2006) |
| Species . | Head width (mm) . | Facet number . | Facet size (µm) . | Rhabdom diameter (µm) . | Activity . | References . |
|---|---|---|---|---|---|---|
| Camponotus consobrinus (major worker) | 3.1 | 798 | 34 | 7.8 | Nocturnal | Narendra et al. (2016b) |
| Camponotus detritus | 3.79 | 1300 | — | — | Diurnal | Menzi (1987) |
| Camponotus irritans | 2.11 | 350 | Nocturnal | Menzi (1987) | ||
| Camponotus ligniperda | 2.48 | 450 | — | — | Nocturnal | Menzi (1987) |
| Camponotus pennsylvanicus (minor worker) | 1.2 | 375 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus pennsylvanicus (major worker) | 3.5 | 658 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus sericeiventris (major worker) | 2.3 | 660 | — | — | Diurnal | R. Nettimi (2017, personal communication) |
| Cataglyphis bicolor (major worker) | 2.1 | 1300 | 24 | 2.5 | Diurnal | Menzi (1987) |
| Formica integroides | 1.9 | 680 | 19.3 | Diurnal | Bernstein and Finn (1971) | |
| Formica polyctena | 1.56 | 750 | 18.4 | 2.6 | Diurnal | Menzel and Blakers (1975) |
| Gigantiops destructor | 2.52 | 4100 | Diurnal | Gronenberg (2008) | ||
| Harpegnathos saltator | 2.2 | 1600 | 30 | 2.2 | Diurnal | A. Narendra (2016, personal observation) |
| Iridomyrmex purpureus | 2 | 496 | 14 | 2.3 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus bagoti (major worker) | 3.2 | 590 | 19 | 1.6 | Diurnal | Schwarz et al. (2011) |
| Melophorus hirsutus (major worker) | 1.7 | 296 | 19 | 1.4 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus hirsutus (minor worker) | 0.98 | 241 | 18 | 1.7 | Diurnal | Ramirez-Esquivel (2012) |
| Myrmecia croslandi | 2.04 | 2363 | 22 | 1.3 | Diurnal | Narendra et al. (2011) |
| Myrmecia desertorum (major worker) | 4.4 | 3625 | 38 | 5.8 | Nocturnal | A. Narendra (2016, personal observation) |
| Myrmecia nigriceps (major worker) | 3.6 | 3210 | 32.9 | 5.5 | Nocturnal | Narendra et al. (2011) |
| Myrmecia piliventris | 1.9 | 2288 | 22 | 1.3 | Diurnal | A. Narendra (2016, personal observation) |
| Myrmecia pyriformis (major worker) | 4.45 | 3593 | 37.5 | 5.9 | Nocturnal | Narendra et al. (2011) |
| Myrmecia pyriformis (small worker) | 2.3 | 2320 | 26 | 5.1 | Nocturnal | Greiner et al. (2007) |
| Myrmecia tarsata (major worker) | 3.7 | 2724 | 28.7 | 2.9 | Nocturnal | Narendra et al. (2011) |
| Nothomyrmecia macrops | 1.6 | 670 | 24.5 | 8.9 | Nocturnal | A. Narendra (2016, personal observation) |
| Notoncus ectatommoides | 1.3 | 209 | 26 | 5.9 | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (major worker) | 0.87 | 27 | 26 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (minor worker) | 0.42 | 21 | 20 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Polyrhachis sokolova | 1.93 | 596 | 33 | 5.0 | Nocturnal | Narendra et al. (2013a) |
| Solenopsis invicta (major worker) | 1.15 | 92.65 | 20.1 | — | Diurnal | Baker and Ma (2006) |
| Solenopsis invicta (minor worker) | 0.58 | 48 | 15.7 | — | Diurnal | Baker and Ma (2006) |
Exclusively day-active animals are classified as diurnal; exclusively nocturnal, crepuscular, and species that are active both during and night are classified as nocturnal.
Summary of known facet numbers, facet diameters, and rhadbom diameters of ant species relative to their head width and activity time
| Species . | Head width (mm) . | Facet number . | Facet size (µm) . | Rhabdom diameter (µm) . | Activity . | References . |
|---|---|---|---|---|---|---|
| Camponotus consobrinus (major worker) | 3.1 | 798 | 34 | 7.8 | Nocturnal | Narendra et al. (2016b) |
| Camponotus detritus | 3.79 | 1300 | — | — | Diurnal | Menzi (1987) |
| Camponotus irritans | 2.11 | 350 | Nocturnal | Menzi (1987) | ||
| Camponotus ligniperda | 2.48 | 450 | — | — | Nocturnal | Menzi (1987) |
| Camponotus pennsylvanicus (minor worker) | 1.2 | 375 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus pennsylvanicus (major worker) | 3.5 | 658 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus sericeiventris (major worker) | 2.3 | 660 | — | — | Diurnal | R. Nettimi (2017, personal communication) |
| Cataglyphis bicolor (major worker) | 2.1 | 1300 | 24 | 2.5 | Diurnal | Menzi (1987) |
| Formica integroides | 1.9 | 680 | 19.3 | Diurnal | Bernstein and Finn (1971) | |
| Formica polyctena | 1.56 | 750 | 18.4 | 2.6 | Diurnal | Menzel and Blakers (1975) |
| Gigantiops destructor | 2.52 | 4100 | Diurnal | Gronenberg (2008) | ||
| Harpegnathos saltator | 2.2 | 1600 | 30 | 2.2 | Diurnal | A. Narendra (2016, personal observation) |
| Iridomyrmex purpureus | 2 | 496 | 14 | 2.3 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus bagoti (major worker) | 3.2 | 590 | 19 | 1.6 | Diurnal | Schwarz et al. (2011) |
| Melophorus hirsutus (major worker) | 1.7 | 296 | 19 | 1.4 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus hirsutus (minor worker) | 0.98 | 241 | 18 | 1.7 | Diurnal | Ramirez-Esquivel (2012) |
| Myrmecia croslandi | 2.04 | 2363 | 22 | 1.3 | Diurnal | Narendra et al. (2011) |
| Myrmecia desertorum (major worker) | 4.4 | 3625 | 38 | 5.8 | Nocturnal | A. Narendra (2016, personal observation) |
| Myrmecia nigriceps (major worker) | 3.6 | 3210 | 32.9 | 5.5 | Nocturnal | Narendra et al. (2011) |
| Myrmecia piliventris | 1.9 | 2288 | 22 | 1.3 | Diurnal | A. Narendra (2016, personal observation) |
| Myrmecia pyriformis (major worker) | 4.45 | 3593 | 37.5 | 5.9 | Nocturnal | Narendra et al. (2011) |
| Myrmecia pyriformis (small worker) | 2.3 | 2320 | 26 | 5.1 | Nocturnal | Greiner et al. (2007) |
| Myrmecia tarsata (major worker) | 3.7 | 2724 | 28.7 | 2.9 | Nocturnal | Narendra et al. (2011) |
| Nothomyrmecia macrops | 1.6 | 670 | 24.5 | 8.9 | Nocturnal | A. Narendra (2016, personal observation) |
| Notoncus ectatommoides | 1.3 | 209 | 26 | 5.9 | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (major worker) | 0.87 | 27 | 26 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (minor worker) | 0.42 | 21 | 20 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Polyrhachis sokolova | 1.93 | 596 | 33 | 5.0 | Nocturnal | Narendra et al. (2013a) |
| Solenopsis invicta (major worker) | 1.15 | 92.65 | 20.1 | — | Diurnal | Baker and Ma (2006) |
| Solenopsis invicta (minor worker) | 0.58 | 48 | 15.7 | — | Diurnal | Baker and Ma (2006) |
| Species . | Head width (mm) . | Facet number . | Facet size (µm) . | Rhabdom diameter (µm) . | Activity . | References . |
|---|---|---|---|---|---|---|
| Camponotus consobrinus (major worker) | 3.1 | 798 | 34 | 7.8 | Nocturnal | Narendra et al. (2016b) |
| Camponotus detritus | 3.79 | 1300 | — | — | Diurnal | Menzi (1987) |
| Camponotus irritans | 2.11 | 350 | Nocturnal | Menzi (1987) | ||
| Camponotus ligniperda | 2.48 | 450 | — | — | Nocturnal | Menzi (1987) |
| Camponotus pennsylvanicus (minor worker) | 1.2 | 375 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus pennsylvanicus (major worker) | 3.5 | 658 | — | — | Nocturnal | Klotz et al. (1992) |
| Camponotus sericeiventris (major worker) | 2.3 | 660 | — | — | Diurnal | R. Nettimi (2017, personal communication) |
| Cataglyphis bicolor (major worker) | 2.1 | 1300 | 24 | 2.5 | Diurnal | Menzi (1987) |
| Formica integroides | 1.9 | 680 | 19.3 | Diurnal | Bernstein and Finn (1971) | |
| Formica polyctena | 1.56 | 750 | 18.4 | 2.6 | Diurnal | Menzel and Blakers (1975) |
| Gigantiops destructor | 2.52 | 4100 | Diurnal | Gronenberg (2008) | ||
| Harpegnathos saltator | 2.2 | 1600 | 30 | 2.2 | Diurnal | A. Narendra (2016, personal observation) |
| Iridomyrmex purpureus | 2 | 496 | 14 | 2.3 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus bagoti (major worker) | 3.2 | 590 | 19 | 1.6 | Diurnal | Schwarz et al. (2011) |
| Melophorus hirsutus (major worker) | 1.7 | 296 | 19 | 1.4 | Diurnal | Ramirez-Esquivel (2012) |
| Melophorus hirsutus (minor worker) | 0.98 | 241 | 18 | 1.7 | Diurnal | Ramirez-Esquivel (2012) |
| Myrmecia croslandi | 2.04 | 2363 | 22 | 1.3 | Diurnal | Narendra et al. (2011) |
| Myrmecia desertorum (major worker) | 4.4 | 3625 | 38 | 5.8 | Nocturnal | A. Narendra (2016, personal observation) |
| Myrmecia nigriceps (major worker) | 3.6 | 3210 | 32.9 | 5.5 | Nocturnal | Narendra et al. (2011) |
| Myrmecia piliventris | 1.9 | 2288 | 22 | 1.3 | Diurnal | A. Narendra (2016, personal observation) |
| Myrmecia pyriformis (major worker) | 4.45 | 3593 | 37.5 | 5.9 | Nocturnal | Narendra et al. (2011) |
| Myrmecia pyriformis (small worker) | 2.3 | 2320 | 26 | 5.1 | Nocturnal | Greiner et al. (2007) |
| Myrmecia tarsata (major worker) | 3.7 | 2724 | 28.7 | 2.9 | Nocturnal | Narendra et al. (2011) |
| Nothomyrmecia macrops | 1.6 | 670 | 24.5 | 8.9 | Nocturnal | A. Narendra (2016, personal observation) |
| Notoncus ectatommoides | 1.3 | 209 | 26 | 5.9 | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (major worker) | 0.87 | 27 | 26 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Pheidole sp. (minor worker) | 0.42 | 21 | 20 | — | Nocturnal | Ramirez-Esquivel (2012) |
| Polyrhachis sokolova | 1.93 | 596 | 33 | 5.0 | Nocturnal | Narendra et al. (2013a) |
| Solenopsis invicta (major worker) | 1.15 | 92.65 | 20.1 | — | Diurnal | Baker and Ma (2006) |
| Solenopsis invicta (minor worker) | 0.58 | 48 | 15.7 | — | Diurnal | Baker and Ma (2006) |
Exclusively day-active animals are classified as diurnal; exclusively nocturnal, crepuscular, and species that are active both during and night are classified as nocturnal.
Regulating light flux to occupy wider temporal niches
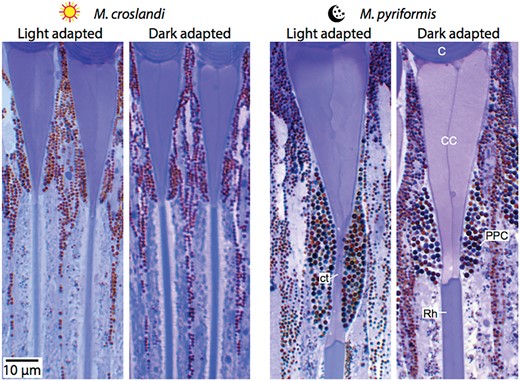
Very few ants are exclusively nocturnal. A majority of them switch activity from day to night based on their temperature preference (e.g., Duncan and Crewe 1994) or tidal patterns (Narendra et al. 2013a). These ants, similar to the exclusively nocturnal ants, have large facets and wide rhabdoms (Narendra et al. 2013a). How do they cope with changes in light intensity? Even the strictly nocturnal ants have to cope with such changes in light intensity when their nests are disturbed during the day and workers have to rush out to defend their nest. Ants that encounter a wide range of light intensities regulate the amount of light that reaches the rhabdom through a pupillary mechanism (Menzi 1987; Narendra et al. 2013a, 2016a). When exposed to bright light, these ants have a variable primary pigment cell that constricts the crystalline cone to form a narrow cone tract (<1 µm in diameter) to control light flux (Fig. 4). When the eye is dark-adapted the primary pigment cells move away from the crystalline cone, increasing the diameter of the proximal crystalline cone to >5 µm. While the constriction is light dependent, the opening of the aperture in the dark is controlled by an endogenous rhythm (Narendra et al. 2016a). This primary pigment cell-driven pupillary mechanism is absent in the strictly day-active ants such as C. bicolor, F. polyctena, and M. croslandi (Brunnert and Wehner 1973; Menzel and Knaut 1973; Menzi 1987; Narendra et al. 2016a). In these day-active ants, as a response to bright light, only the retinula cell pigment granules migrate radially toward the rhabdom in the light-adapted state and they move away from the rhabdom in the dark-adapted state. Thus, the pupillary mechanisms allow ants to modify their optical sensitivity to be active in a wide range of light intensities.
Longitudinal sections of ommatida show that a light-dependent pupillary mechanism is present in nocturnal ants. Eyes of the day- (left) and night-active ants (right) were light-adapted (300 lux from 3 h after sunrise for 24 h) or dark-adapted (stored in light-tight container from 3 h after sunset for 24 h). In the night-active species the primary pigment cell pupil constricts the crystalline cone to form a narrow cone tract of 1.6 µm in diameter in the light-adapted state. In the dark-adapted state, the primary pigment cell pupil moves away from the crystalline cone increasing the diameter of the proximal crystalline cone to 5.3 µm. Such migration of the primary pigment cells does not occur in the day-active species. Modified from Narendra et al. (2016a). Cornea (C), crystalline cone (CC), primary pigment cell (PPC), crystalline cone tract (ct), and rhabdom (Rh) are shown.
Investigating the visual system of ants is further rewarding due to differences in locomotion and activity time within a single species. Workers of M. pyriformis restrict their activity to the dim-light periods of twilight and night, whereas alates fly out of the nest in the day to mate (Narendra et al. 2011). Following mating, the now inseminated female establishes a nest of her own and forages during the early evening for a brief period of her life. The nocturnal workers have a visual system designed for dim-light, diurnal males have a visual system designed for life on the wing and for diurnal activity, and winged females have an intermediate visual system best suited for diurnal flying and pedestrian foraging in dim-light (Narendra et al. 2011). In leaf cutter ants (Atta spp.), the nocturnal alates have significantly larger facets compared with the diurnal alates (Moser et al. 2004). Thus, both between and within species, visual systems have evolved to suit specific light environments. Such intraspecific visual adaptations are also found in bees and wasps (Greiner 2006; Somanathan et al. 2009). These adaptations alone, however, are not sufficient to explain the visually guided behavior of insects at light intensities that are up to 11 orders of magnitude dimmer than those during the day. To further suppress noise and to improve the visual signal, insects have developed physiological means of improving optical sensitivity by sacrificing spatial and temporal resolution (Warrant et al. 1996; Warrant 1999, 2008, 2017; Warrant and Dacke 2010, 2016; Stöckl et al. 2016a).
How fast are photoreceptors of nocturnal ants?
Temporal resolution is the ability of an eye to detect fast moving objects and is typically identified by the impulse response, the photoreceptor response time to a brief flash of light. In ants, photoreceptor response times have been studied not according to the light levels that animals experience but in the context of walking speeds. The fast moving Pseudomyrmex phyllophilus has faster responses (ca. 15 ms) to a 300 ms flash of light compared with the slow-moving Camponotus rufipes and Atta sexdens (de Souza and Ventura 1989). In bees, the nocturnal Megalopta genalis encodes less information at both bright and dim-light levels compared with the diurnal Lassioglossum leucozonium (Frederiksen et al. 2008). This difference is because their photoreceptors have a low signal-to-noise ratio and thus a higher optical sensitivity, which is a clear visual adaptation among nocturnal insects. Nocturnal ants, in addition, have slow UV photoreceptors (Ogawa et al. 2015). UV sensitivity (average sensitivity between 350 and 400 nm) dropped by 42.2% when the stimulation frequency increased from 10 to 30 Hz. This reduced sensitivity at short wavelengths in response to higher stimulation frequency was also present in diurnal ants, but the effect was much smaller. UV receptors in nocturnal ants are thus comparatively slow, which might selectively increase sensitivity to short wavelengths. Indeed, the ratio of the spectral sensitivity at short wavelengths to peak sensitivity is relatively high in the nocturnal ants. This might be an adaptation to ensure the UV signal is reliable at low light intensities which is crucial for visual navigation as it increases the contrast between the sky and the ground in the visual panorama (Wilson 1978; Moller 2002; Schultheiss et al. 2016). The UV range is also used to obtain reliable compass information from the pattern of polarized skylight (Zeil et al. 2014).
Ants have long been considered unique among hymenopterans in being dichromats (sensitive to UV and green wavelengths) (Menzel and Knaut 1973; Lieke 1981; Labhart 1986; Cammaerts 2007). Recent evidence suggests that both diurnal and nocturnal ants have the molecular (Yilmaz et al. 2016) and physiological basis (Ogawa et al. 2015) for trichromacy. Intracellular recordings in nocturnal bull ants Myrmecia vindex have shown that their compound eye is sensitive to UV, blue, and green regions of the spectrum. Why do ants need color vision? We know color vision is useful for reliable object discrimination because it is not affected by shifts in intensity or ambient light conditions (Johnsen et al. 2006; Somanathan et al. 2008; Kinoshita and Arikawa 2014). However, at present there is no clear behavioral evidence to demonstrate the function of color vision in ants. Since many hymenopterans rely on pollen and nectar offered by plants, it is tempting to assume that insect visual sensory system is adapted to detect floral patterns. However, trichromacy in arthropods predates the evolution of angiosperms (Chittka 1996), suggesting that color vision may have evolved for object detection, which is an integral part of navigation (Ogawa et al. 2015). Both behavioral and physiological investigation are needed to elucidate the function of color vision in ants.
Spatial summation
There is growing evidence in several insects that spatial summation occurs in the lamina, the first visual processing neuropil (e.g., Greiner et al. 2004; Stöckl et al. 2016a, 2016b). The retinal axons of a single ommatidium project to one cartridge in the lamina where the cartridges are retinotopically organized. In addition to the short and long visual fibers, the lamina contains relay neurons, the laminar monopolar cells (LMCs), that receive input from photoreceptor axons. In nocturnal insects, the dendrites of the LMCs extend into neighboring cartridges whereas in the diurnal insects they are confined to their own cartridge. Such dendritic branching is thought to improve spatial summation in insects active in dimly lit habitats. Differences in dendritic branching have been documented in nocturnal and diurnal bees (Greiner et al. 2005) and hawkmoths (Stöckl et al. 2016b). In ants, the extent of branching has been documented only in a day-active ant C. bicolor (Ribi 1975), in which LMCs are confined to their own cartridge. To the best of our knowledge, this has not been investigated or characterized in nocturnal ants.
Conclusion
Ants occupy a wide range of niches, from the dimmest to the brightest terrestrial habitats. Ants active in dimly lit habitats use navigational strategies similar to diurnal ants such as deriving visual compass information from both celestial and terrestrial cues. Nocturnal ants use both local and distant terrestrial landmark information to navigate through their cluttered world. Even in low light conditions, their visual system is sensitive enough to detect subtle changes in their familiar landmark panorama. However, as light intensity decreases during the night accessing visual navigational information becomes difficult and the navigational competence of ants also decreases. Hence, visually guided nocturnal ants carry out a majority of their navigational tasks during the twilight period. To improve the visual reliability in low light conditions they have evolved distinct optical adaptations that include large facets and wide rhabdoms together with slow photoreceptor response time. Studies of the investment patterns in brain regions involved in visual processing would further elucidate adaptations for dim-light activity.
Acknowledgments
The authors thank Zachary Sheehan and Fiorella Ramirez-Esquivel for several discussions and for their help during the preparation of this manuscript. They are eternally grateful to several of their colleagues, especially Eric Warrant, Jochen Zeil, Willi Ribi, and Wulfila Gronenberg, whose work has inspired them to understand the visual systems of nocturnal ants.
Funding
This work was supported by grants from the Australian Research Council [DE120100019, FT140100221, DP150101172]. The authors are also grateful for financial support from the Society of Integrative and Comparative Biology and from the Company of Biologists.
References
Author notes
From the symposium “Low Spatial Resolution Vision–Function and Evolution” presented at the annual meeting of the Society for Integrative and Comparative Biology, January 4–8, 2017 at New Orleans, Louisiana.